PRODUCT IDENTIFICATION
H.S. CODE
TOXICITY
CLASSIFICATION
PHYSICAL AND CHEMICAL PROPERTIES
AUTOIGNITION
NFPA RATINGS
REFRACTIVE INDEX
Stable under ordinary conditions
GENERAL DESCRIPTION AND APPLICATIONS
APPEARANCE
ASSAY
|
|
|
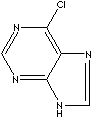
| 6-CHLOROPURINE | ||
|
PRODUCT IDENTIFICATION |
||
| CAS NO. | 87-42-3 |
|
| EINECS NO. | 201-745-6 | |
| FORMULA | C5H3ClN4 | |
| MOL WT. | 154.56 | |
|
H.S. CODE |
||
|
TOXICITY |
|
|
| SYNONYMS | 6-Chloro-9H-Purine; | |
| SMILES |
|
|
|
CLASSIFICATION |
||
|
PHYSICAL AND CHEMICAL PROPERTIES |
||
| PHYSICAL STATE | light yellow powder | |
| MELTING POINT | > 300 C | |
| BOILING POINT | ||
| SPECIFIC GRAVITY | ||
| SOLUBILITY IN WATER | ||
| pH | ||
| VAPOR DENSITY |
|
|
|
AUTOIGNITION |
|
|
|
NFPA RATINGS |
Health: 1; Flammability: 0; Instability: 0 | |
|
REFRACTIVE INDEX |
|
|
| FLASH POINT | ||
| STABILITY |
Stable under ordinary conditions |
|
|
GENERAL DESCRIPTION AND APPLICATIONS |
||
| Purine is a heterocyclic compound featured by a fused pyrimidine and imidazole rings composed of carbon and nitrogen atoms. The simplest one is purine itself and the two major purines are adenine(6-Aminopurine) and guanine (2-Amino-6-hydroxypurine) which are two bases components of nucleic acid and the nucleotides. Purine itself is not found in nature, but as substituted purines such as methyled, hydroxyl and amino substituted. In addition to adenine and guanine, a group of chemical compounds called purine base include hypoxanthine (6-oxypurine), xanthine (2,6-dioxypurine), uric acid (2,6,8-trioxypurine), and theobromine (3,7-dimethyl xanthine). Theophylline and caffeine are a member of methylated purine family. Purines are biologically important in In medicine and biological research. | ||
| SALES SPECIFICATION | ||
|
APPEARANCE |
light yellow powder | |
|
ASSAY |
99.0% min | |
| MELTING POINT | > 300 C | |
| TRANSPORTATION | ||
| PACKING |
|
|
| HAZARD CLASS | Not regulated | |
| UN NO. | ||
| OTHER INFORMATION | ||
| Hazard Symbols: , Risk Phrases: 22-36/37/38, Safety Phrases: 26-36 | ||
|
|
|