PRODUCT IDENTIFICATION
103-90-2
H.S. CODE
TOXICITY
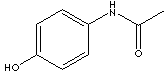
Paracetamole; P-acetamido-Phenol; 4'-hydroxyacetanilide; n-(p- Hydroxyphenyl)-Acetamide; N-(4-hydroxyphenyl)-Acetamide; P-acetamidophenol; 4-Acetamidophenol; Acetaminofen; Acetaminophen; P- Acetaminophenol; N-acetyl-p-aminophenol; P-Acetylamino Phenol; P-hydroxyacetanilide; Paracetamol; 4-hydroxy Acetanilide; 4-hydroxyanilid Kyseliny Octove; N-(4-hydroxyphenyl) Acetamide;
p-aminophenol, acetic anhydride
CLASSIFICATION
PHYSICAL AND CHEMICAL PROPERTIES
white crystalline powder
5.5 - 6.5
AUTOIGNITION
NFPA RATINGS
REFRACTIVE INDEX
Stable under ordinary conditions
GENERAL DESCRIPTION & APPLICATIONS
Acetaminophen is an odorless, slightly bitter taste white crystalline powder. It is soluble in organic solvents such as methanol and ethanol but slightly soluble in water and ether. It's pH range is 5.5 - 6.5 based on saturated aqueous solution. It, chemically N-(4-Hydroxyphenyl) acetamide, is derived from the interaction of p-aminophenol and an aqueous solution of acetic anhydride. Acetaminophen is a nonprescription analgesic and antipyretic drug similar to aspirin. But acetaminophen is not an NSAID (Nonsteroidal Antiinflammatory Drug) as it doesn't participate in the inflammatory response. But acetaminophen is not an NSAID as it doesn't participate in the inflammatory response as it can not inhibit cyclooxygenases in the presence of peroxides. There are high levels of peroxides in platelets. Prostaglandin which reduces blood coagulation is produced through cyclooxygenase pathway. In result, NSAIDs including aspirin have detrimental effects on the stomach lining, where prostaglandins serve a protective role, but paracetamol has almost no adverse effects on the stomach or esophagus. Taking large doses of acetaminophen for a long time may impair liver function to some liver damage. Paracetamol (para-acetyl-amino-phenol) is another name of acetaminophen (N-acetyl-para-aminophenol). It is also used as an intermediate for pharmaceuticals(as a precursor in penicillin) and azo dyes, stabilizer for hydrogen peroxide, photographic chemicals.
BIBLIOGRAPHY
BP93 / USP23
APPEARANCE
white crystalline powder
IDENTIFICATION
complies
ASSAY
99.0-101.0%
MELTING POINT
168 - 172 C
HEAVY METAL
10ppm max
P-AMINOPHENOL
50ppm max
RESIDUE ON IGNITION
0.1% max
LOSS ON DRYING
0.5% max
CHLORIDE
0.014% max
SULFATE
0.02% max
SULFIDE
pass test
READILY CARBONIZABALES
pass test (4-chloroacetanilide)
and ACETAMINOPHEN DC 90
Nonsteroidal Antiinflammatory Drugs (NSAIDs); chemically heterogeneous large groups of drugs which suppress inflammation in a manner similar to steroids, but less side effects of sedation, respiratory depression, or addiction than steroids. They are widely used for the treatment of inflammatory disorders and painful conditions such as rheumatoid arthritis, gout, bursitis, painful menstruation, and headache. They are effective in the relief of pain and fever. NSAIDs inhibit the cyclooxygenase (COX) activity resulting in decreased synthesis of prostaglandin, leukotriene and thromboxane precursors such as the ubiquitous enzyme which catalyzes the initial step in the synthesis of prostanoids. Prostanoid is any of a group of C-20 fatty acids complex with an internal five or six carbon rings such as prostaglandins, prostanoic acid, prostacyclins, and thromboxane; derived from arachidonic acid (C-20 polyunsaturated fatty acid with four cis double bonds). The action or the synthesis of prostanoids are involved in the modulation of a variety of pathophysiologic processes including inflammation, hemostasis, thrombosis, cytoprotection, ulceration, hemodynamics and other the progression of kidney diseases. Thus, NSAIDs as non-selective inhibitors of the cyclooxygenases (both the cyclooxygenase-1 and cyclooxygenase-2 isoenzymes) may have beneficial as well as untoward effects on a variety of human diseases. Low stomach prostanoid levels caused by COX-1 inhibitors can result in ulceration and internal bleeding and perforation. The selective COX-2 inhibitors such as oxicam, meloxicam, and coxibs (celecoxib, rofecoxib, valdecoxib, parecoxib and etoricoxib) do not interfere with COX-1. The most prominent NSAID is aspirin. Nonaspirin NSAIDs can be classified based on chemical structures.
Nonsteroidal Anti-Inflammatory Drugs (NSAID) by chemical structure
- Carboxylic Acid Groups
- Salicylates (Acetylsalicylate, Choline salicylate, Diflunisal, Magnesium choline salicylate, Magnesium salicylate, Salsalate)
- Acetic Acids (Bendazac, Diclofenac, Etodolac, Indomethacin, Ketorolac, Nabumetone, Sulindac, Tolmetin)
- Propionic acids (Carprofen, Fenoprofen, Flurbiprofen, Ibuprofen, Ketoprofen, Loxoprofen, Naproxen, Naproxen sodium, Oxaprozin, Vedaprofen)
- Anthranilic acids (Meclofenamic acid, Meclofenamate sodium, Tolfenamic acid)
- Phenylacetic acids
- Aminonicotinic acids (Flunixin)
- Indole Analogs (Indomethacin, Nabumetone, Ketorolac, Etodolac,)
- Enolic Acid Groups (which doesn't have carboxylic group but acid due to the enolic hydroxy substituent)
- Pyrazolones (Phenylbutazone, Oxyphenbutazone, Dipyrone, Ramifenazone)
- Oxicams (Meloxicam, Piroxicam, Tenoxicam)
- Coxibs
- Celecoxib, Rofecoxib, Valdecoxib, Parecoxib, Etoricoxib
- Gold Salts
- Auranofin, Gold sodium thiomalate, Aurothioglucose