ACETYLSALICYLIC ACID
PRODUCT IDENTIFICATION
50-78-2
H.S. CODE
TOXICITY
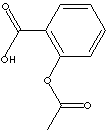
ASPIRIN; 2-(acetyloxy)-Benzoic acid; Solpyron; Ecotrin;
CLASSIFICATION
GENERAL DESCRIPTION
Acetylsalicylic Acid, also known by trade name Aspirin, is an acetyl derivative of salicylic acid that is a white, crystalline, weakly acidic substance, with melting point 137°C. It is useful in the relief of headache and muscle and joint aches. Aspirin is also effective in reducing fever, inflammation, and swelling and thus has been used for treatment of rheumatoid arthritis, rheumatic fever, and mild infection. Large doses cause acid-base imbalance and respiratory disturbances and can be fatal, especially in children. Acetaminophen (known by trade name Tylenol), which does not cause gastric irritation but does lower fever and relieve pain, is often substituted for Aspirin.
PHYSICAL AND CHEMICAL PROPERTIES
Odorless, Colourless or a white crystalline powder
136 C (with decomposition)
140 C
1.35
AUTOIGNITION
NFPA RATINGS
REFACTIVE INDEX
APPLICATIONS
Acetylsalicylic Acid is used in analgesics, anti-inflammatories, antipyretics, anticoagulants and anti-rheumatics. It is also used as an additive in food, animal feed, drug and cosmetic.
Nonsteroidal Antiinflammatory Drugs (NSAIDs); chemically heterogeneous large groups of drugs which suppress inflammation in a manner similar to steroids, but less side effects of sedation, respiratory depression, or addiction than steroids. They are widely used for the treatment of inflammatory disorders and painful conditions such as rheumatoid arthritis, gout, bursitis, painful menstruation, and headache. They are effective in the relief of pain and fever. NSAIDs inhibit the cyclooxygenase (COX) activity resulting in decreased synthesis of prostaglandin, leukotriene and thromboxane precursors such as the ubiquitous enzyme which catalyzes the initial step in the synthesis of prostanoids. Prostanoid is any of a group of C-20 fatty acids complex with an internal five or six carbon rings such as prostaglandins, prostanoic acid, prostacyclins, and thromboxane; derived from arachidonic acid (C-20 polyunsaturated fatty acid with four cis double bonds). The action or the synthesis of prostanoids are involved in the modulation of a variety of pathophysiologic processes including inflammation, hemostasis, thrombosis, cytoprotection, ulceration, hemodynamics and other the progression of kidney diseases. Thus, NSAIDs as non-selective inhibitors of the cyclooxygenases (both the cyclooxygenase-1 and cyclooxygenase-2 isoenzymes) may have beneficial as well as untoward effects on a variety of human diseases. Low stomach prostanoid levels caused by COX-1 inhibitors can result in ulceration and internal bleeding and perforation. The selective COX-2 inhibitors such as oxicam, meloxicam, and coxibs (celecoxib, rofecoxib, valdecoxib, parecoxib and etoricoxib) do not interfere with COX-1. The most prominent NSAID is aspirin. Nonaspirin NSAIDs can be classified based on chemical structures.
Nonsteroidal Anti-Inflammatory Drugs (NSAID) by chemical structure
- Carboxylic Acid Groups
- Salicylates (Acetylsalicylate, Choline salicylate, Diflunisal, Magnesium choline salicylate, Magnesium salicylate, Salsalate)
- Acetic Acids (Bendazac, Diclofenac, Etodolac, Indomethacin, Ketorolac, Nabumetone, Sulindac, Tolmetin)
- Propionic acids (Carprofen, Fenoprofen, Flurbiprofen, Ibuprofen, Ketoprofen, Loxoprofen, Naproxen, Naproxen sodium, Oxaprozin, Vedaprofen)
- Anthranilic acids (Meclofenamic acid, Meclofenamate sodium, Tolfenamic acid)
- Phenylacetic acids
- Aminonicotinic acids (Flunixin)
- Indole Analogs (Indomethacin, Nabumetone, Ketorolac, Etodolac,)
- Enolic Acid Groups (which doesn't have carboxylic group but acid due to the enolic hydroxy substituent)
- Pyrazolones (Phenylbutazone, Oxyphenbutazone, Dipyrone, Ramifenazone)
- Oxicams (Meloxicam, Piroxicam, Tenoxicam)
- Coxibs
- Celecoxib, Rofecoxib, Valdecoxib, Parecoxib, Etoricoxib
- Gold Salts
- Auranofin, Gold sodium thiomalate, Aurothioglucose
BIBLIOGRAPHY
BP / USP / JP
APPEARANCE
white to off-white crystalline powder
IDENTITY
Complies
SOLUTION IN ETHANOL
Colourless, Clear
ASSAY
99.5 to 100.5 %
FREE SALICYLIC ACID
0.1 % max
LOSS ON DRYING
0.5 % max
SULPHATED ASH
0.1 % max
HEAVY METALS (as Pb)
20 ppm max
RELATED SUBSTANCES
0.25% max
PARTICLE SIZE
Retained
on 180 µm (50 % maximum)
Retained
on 710 µm (10 % maximum)
25kgs in Fiber Drum
GENERAL DESCRIPTION OF SALICYLIC ACID