PRODUCT IDENTIFICATION
200-389-9
H.S. CODE
TOXICITY
CLASSIFICATION
PHYSICAL AND CHEMICAL PROPERTIES
AUTOIGNITION
NFPA RATINGS
REFRACTIVE INDEX
GENERAL DESCRIPTION AND APPLICATIONS
Adenosine Triphosphate (ATP) : a nucleotide composed of adenine, the sugar ribose, and three phosphate groups; involved in energy metabolism and required for RNA synthesis. It exists in cells in a form of high-energy phosphate bond to store and transport chemical energy. The pyrophosphate nature of the bonds between ATP's three phosphate radicals results in a powerful donor of phosphate groups to suitable acceptors. When it is broken down by hydrolysis, it yields ADP (adenosine diphosphate), inorganic phosphorus, and energy. The free energy derived from hydrolysis of ATP is used to drive metabolic reactions including the synthesis of nucleic acids and proteins, to move molecules against concentration gradients (active transport), and to produce mechanical motion (contraction of microfibrils and microtubules). ADP can be further broken down to yield adenosine monophosphate (AMP), additional phosphorus, and more energy. When the phosphorus and energy are immediately used to drive other reactions, such as the synthesis of UDP (uridine diphosphate), an RNA precursor, from UMP (uridine monophosphate), the pair of reactions are said to be coupled. New ATP is produced from AMP using the energy released from the breakdown of fuel molecules, such as fat and glucose which is broken down into pyruvate in the cytosol. Two molecules of ATP are generated for each molecule of glucose. ADT can be converted back to ATP by the processes of oxidative phosphorylation and substrate-level phosphorylation.
Adenosine Diphosphate (ADP) : a nucleotide composed of pyrophosphate of adenosine, involved in energy metabolism; it is produced by hydrolysis of ATP and converted back to ATP by the processes of oxidative phosphorylation and substrate-level phosphorylation.
Adenosine Monophosphate (AMP, also called adenylic acid.) : a nucleotide, 5'-phosphate of adenosine, produced by the hydrolysis of ATP and converted to ADP by adenylate kinase. Involved in the reactions of intracellular energy transfers.
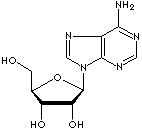
Deoxyadenosine (dA) : a purine nucleoside composed of adenine linked by its N9 nitrogen to the C1 carbon of deoxyribose. (deoxy-, also called desoxy, is a prefix for the designation of compounds which contain one less atom of oxygen than the reference substance).
Deoxyadenosine diphosphate (dADP) : a nucleotide, 5'-pyrophosphate of deoxyadenosine.
Deoxyadenosine monophosphate (dAMP) : a nucleotide, 5'-phosphate of deoxyadenosine, occurring in deoxyribonucleic acid.
Deoxyadenosine triphosphate (dATP): a nucleotide, the 5'-triphosphate of deoxyadenosine; activated precursor in DNA synthesis.
GENERAL DESCRIPTION OF PURINE: Purine is a heterocyclic compound featured by a fused pyrimidine and imidazole rings composed of carbon and nitrogen atoms. The simplest one is purine itself and the two major purines are adenine(6-Aminopurine) and guanine(2-Amino-6-hydroxypurine) which are two bases components of nucleic acid and the nucleotides. Purine itself is not found in nature, but as substituted purines such as methyled, hydroxyl and amino substituted. In addition to adenine and guanine, a group of chemical compounds called purine base include hypoxanthine (6-oxypurine), xanthine (2,6-dioxypurine), uric acid (2,6,8-trioxypurine), and theobromine (3,7-dimethyl xanthine). Theophylline and caffeine are a member of methylated purine family. Purines are biologically important in In medicine and biological research.APPEARANCE
98.0 - 102.0%
OPTICAL ROTATION
-69° ~ -71° (C=2 in 5% NaOH)
HEAVY METALS
10ppm max
0.5% max