| CAS
NO. |
78110-38-0
80951-91-3
149496-40-2 |
|
| EINECS
NO. |
278-839-9 |
| FORMULA |
C13H19N5O8S2 |
| MOL
WT. |
437.441 |
|
H.S.
CODE
|
|
|
TOXICITY
|
|
| SYNONYMS |
Azactam;
|
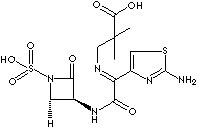
| (Z)-2-[[[(2-amino-4-thiazolyl)[[(2S,-3S)-2-methyl-4-
oxo-1-sulfo-3-azetidinyl]carbamoyl] methylene] amino] oxy]-2-methyl propionic acid;
[2S-[2alpha,3beta(Z)]]-2- [[[1-(2- Amino-4-thiazolyl)- 2-[(2-methyl-4-oxo-1-sulfo-
3-azetidinyl) amino]-2-oxo ethylidene] amino] oxy]-2-methylpropanoic
acid; 2-[(Z-(2)-Aminothiazol-4-yl)-2-[(2S,3S)-2- methyl-4- oxo-1-sulfoazetidin-3-yl
carbamoyl] methyleneaminooxy]-2- methyl-1-propanoic
acid; |
| SMILES |
|
|
CLASSIFICATION
|
|
|
PHYSICAL
AND CHEMICAL PROPERTIES
|
| PHYSICAL
STATE |
white
to off-white
crystals |
| MELTING
POINT |
|
| BOILING
POINT |
|
| SPECIFIC
GRAVITY |
|
| SOLUBILITY
IN WATER |
|
| pH |
|
| VAPOR
DENSITY |
|
|
AUTOIGNITION
|
|
|
NFPA
RATINGS
|
Health:
1; Flammability: 0; Instability: 0 |
|
REFRACTIVE
INDEX
|
|
| FLASH
POINT |
|
| STABILITY |
Stable
under ordinary conditions. |
|
APPLICATIONS
|
|
Aztreonam is a monobactam (monocyclic beta-lactam nucleus) antibiotic. It has
side chains for activity enhencing and stability. It inhibits cell wall
biosynthesis by binding to penicillin-binding protein of aerobic gram-negative
bacilli. It has very active and broad spectrum activity against aerobic-gram
negative bacteria, including Pseudomonas aeruginosa, Hemophilus influenzae,
Enterobacteriaceae, and Neisseria gonorrhea; This spectrum is similar to the
3rd generation cephalosporins or aminoglycosides. As it has limited activity to
only aerobic-gram negative bacteria, it is used in combination with
gram-positive bacteria anaerobes sometimes. It is frequently used in patients
unable to tolerate beta-lactams.It is resistant to beta-lactamases and is useful
against gram-negative pathogens. The chemical designation is
2-[(Z-(2)-Aminothiazol-4-yl)-2-[(2S,3S)-2-methyl-4- oxo-1-sulfoazetidin-3-yl
carbamoyl] methyleneaminooxy]-2- methyl-1-propanoic
acid. It is a white crystalline powder, odorless. Commercial aztreonam is the
mixture with arginine (apprx 4:3). |
| SALES
SPECIFICATION |
|
APPEARANCE
|
white
to off-white
crystals |
|
IDENTIFICATION
|
Complies
|
|
ASSAY
|
90.0%
min
|
| pH |
4.5
- 7.5 |
|
WATER
|
2.0%
max
|
|
STERILITY
|
Sterile
|
|
ARGININE
|
40.0
- 45.0%
|
| TRANSPORTATION |
| PACKING |
|
| HAZARD
CLASS |
|
| UN
NO. |
|
| OTHER
INFORMATION |
| Hazard Symbols: n/a, Risk Phrases: n/a, Safety Phrases:
22-24/25 |
