| CAS
NO. |
91-01-0 |
|
| EINECS
NO. |
202-033-8 |
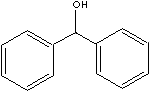
| FORMULA |
(C6H5)2CHOH |
| MOL
WT. |
184.24 |
|
H.S.
CODE
|
2907.29 |
|
TOXICITY
|
Oral rat LD50: 5000
mg/kg |
| SYNONYMS |
Benzohydrol;
Benzhydryl alcohol; Diphenylcarbinol;
|
|
Diphenylmethanol; Alpha-phenyl-Benzenemethanol;
Hydroxydiphenylmethane;
|
| SMILES |
|
|
CLASSIFICATION
|
|
|
PHYSICAL AND CHEMICAL PROPERTIES
|
| PHYSICAL
STATE |
white
flakes |
| MELTING POINT |
65
- 69 C |
| BOILING
POINT |
297
- 299 C |
| SPECIFIC GRAVITY |
|
| SOLUBILITY
IN WATER |
Slightly soluble |
| pH |
|
| VAPOR DENSITY |
|
|
AUTOIGNITION
|
|
|
NFPA
RATINGS
|
Health: 1; Flammability: 0; Reactivity: 0 |
|
REFRACTIVE
INDEX
|
|
| FLASH
POINT |
|
| STABILITY |
Stable
under ordinary conditions |
|
APPLICATIONS
|
|
Benzhydryl compounds contain two benzene rings which substitute hydrogen atoms
in the methane molecule. This structure does not allow the fusion of benzene
ring. This nomenclature system is particularly useful to describe the groups
attached to the benzene rings and the additional substituents on the methane.
The benzhydryl structure features as the motif for modified fibers, polymers and
curing agents. The benzhydryl motif is a fundamental component also in drugs
such antihistamines, antihypertensive agents and antiallergenic agents. Diphenylmethane
structure is a moiety of pesticides such as hexachlorophene
and (CAS RN: 70-30-4) and dichlorophen
(CAS RN: 97-23-4). The
term "trityl " refers to a triphenylmethanyl
compound in which three phenyl rings are bonded to a single carbon.
Trityl anion stabilized by extensive delocalization over three
phenyl rings absorbs the visible region of the spectrum and originates brilliant and fluorescent
red to violet colors. Triphenylmethane is the
basic skeleton of many dyes. Benzhydrol
is used as an intermediate of pharmaceuticals (including
antihistamines),
agrochemicals and other organic compounds.
It is used as a fixative in perfumery and
as a terminating group in polymerizations.
Benzhydyl is a skeleton for Histamine H1 antagonist which an ethylamine group is
attached to a diphenylmethane structure. Common examples are diphenhydramine, diphenidol and
phenyltoloxamine.
|
| SALES
SPECIFICATION |
|
APPEARANCE
|
white
flakes |
|
ASSAY
|
99.0%
min
|
|
MELTING POINT |
65
- 69 C |
|
LOSS
ON DRYING
|
0.5%
max
|
|
RESIDUE
ON IGNITION
|
0.1%
max
|
| TRANSPORTATION |
| PACKING |
25kgs
in fiber drum |
| HAZARD CLASS |
Not
regulated |
| UN
NO. |
|
| OTHER
INFORMATION |
|
Hazard
Symbols: n/a, Risk Phrases: n/a, Safety Phrases: 28A/37/37/45 |
