BOP REAGENT
PRODUCT IDENTIFICATION
56602-33-6
442.28
H.S. CODE
TOXICITY
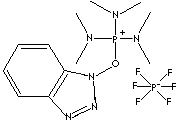
Benzotriazol-1-yloxy-tris(dimethylamino)-phosphonium hexafluorophosphate; Phosphorus(1++), (1-hydroxy-1H-benzotriazolato-O)tris(N-methylmethanaminato)-, (beta-4)-, hexafluorophosphate(1-); Benzotriazol-1-yloxy tris (dimethylamino)-phosphonium hexafluorophosphate; Tri(dimethylamino) benzotriazol- 1-yloxyphosphonium hexafluorophosphate; (Benzotriazol-1-yloxy) tris(dimethylamino) phosphonium hexafluorophosphate;
SMILES
[P+](On1nnc2c1cccc2)(N(C)C)(N(C)C)N(C)C.[P-](F)(F)(F)(F)(F)F
CLASSIFICATION
Coupling reagent,
EXTRA NOTES
The substance or mixture is a flammable solid with the category 1.
PHYSICAL AND CHEMICAL PROPERTIES
AUTOIGNITION
NFPA RATINGS
Health hazard: 2, Flammability: 3, Physical hazards: 3
REFRACTIVE INDEX
Stable under ordinary conditions.
EXTERNAL LINKS & GENERAL DESCRIPTION
Wikipedia Linking - BOP reagent
Google Scholar Search - BOP reagent
PubChem Compound Summary - Bop reagent
http://www.ncbi.nlm.nih.gov/ - BOP reagent
Local:
Applications:
Coupling reagent for peptide synthesis, which represses enantiomerization. It is an useful agent for lactonization, selective esterification or amidation of alpha-amino acids without racemization. (Reagent for Peptide coupling, Synthesis of esters, Esterification of carboxylic acids, Plasmid CAN-encapsulating liposomes, Synthesis of magnolamide for antioxidative activity, Catalyst for preparation of 9-acridinecaroboxamide derivative)
Carbodiimides are a group of organic compounds which have the resonance formula N=C=N. Carbodiimide is formed by dehydration of urea or from thiourea. Carbodiimides are readily reacts with various form of amines and hydroxyl functional roups. Carbodiimides are used as dehydration agents and as activating agent of carboxylic acids to form esters or amides. The disubstituted carboxyl activating agents are used for crosslinking proteins to nucleic acids and formation of immunoconjugates in peptide synthesis. N,N'-dicyclohexylcarbodiimide (DCC) is one of the most common peptide coupling agents. It is a low melting point waxy solid; insoluble in water but highly soluble in common organic solvents like acetonitrile dichloromethane, dimethylformamide, and tetrahydrofuran. DCC is a potent allergen. N,N'-diisopropylcarbodiimide (DIC) is an alternative to DCC, DIC is a liquid and is not an allergen. 1-Ethyl-3-(3-dimethylaminopropyl)carbodiimide (EDC) is a water-soluble activating agent for amide bonding with primary amines. It also activates phosphate groups. Typically, it is utilized in the pH range 4.0-6.0 without buffers. In particular, amine and carboxylate buffers should be avoided. Carbodiimides are so active and cause racemization of the amino acid. Active esters are less reactive and less in danger of racemization. 1-Hydroxybenzotriazole (HOBt) and 1-hydroxy-7-aza-benzotriazole (HOAt) are substances that react with the O-acylurea to form active esters. HOAt is a condensation additive in peptide synthesis. It efficiently speeds up coupling process, reduces the loss of chiral integrity, and provides a visual indication (yellow to colorless) of the reaction course. Other active esters exit as non-nucleophilic anionic salts of uronium or phosphonium such as O-(Benzotriazol-1-yl)-N,N,N',N'-tetramethyluronium hexafluorophosphate(HBTU), O-(7-Azabenzotriazol-1-yl)-N,N,N',N'-tetramethyluronium hexafluorophosphate (HATU), (Benzotriazol-1-yloxy)tripyrrolidinophosphonium hexafluorophosphate (PyBOP).
|
|
APPEARANCE
PURITY (HPLC)
99.0% min
ca 138 C
HAZARD OVERVIEW
Flammable solid. Causes skin irritation. May cause respiratory irritation.
GHS
Danger
PICTOGRAMS


HAZARD STATEMENTS
H228-H315-H335
P STATEMENTS
P210-P261
![]()
![]()
RISK PHRASES
2-11-37/38-44
SAFETY PHRASES
35