PRODUCT IDENTIFICATION
H.S. CODE
TOXICITY
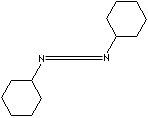
C1CCC(CC1)N=C=NC2CCCCC2
CLASSIFICATION
EXTRA NOTES
A carbodiimide that is used as a chemical intermediate and coupling agent in peptide synthesis. (From Hawley's Condensed Chemical Dictionary, 12th ed)
PHYSICAL AND CHEMICAL PROPERTIES
AUTOIGNITION
NFPA RATINGS
REFRACTIVE INDEX
113 C
EXTERNAL LINKS & GENERAL DESCRIPTION
Drug Information Portal (U.S. National Library of Medicine) - Dicyclohexylcarbodiimide
PubChem Compound Summary - Dicyclohexylcarbodiimide
http://www.ebi.ac.uk/ - Dicyclohexylcarbodiimide
http://www.ncbi.nlm.nih.gov/ - Dicyclohexylcarbodiimide
http://www.bgu.ac.il/
Carbodiimide
reagents were designed to prevent the formation of the undesired
N-acylurea and to facilitate easy separation from the by-products.
The insolubility of the by-product occasionally caused problems
for the synthesis of polypeptides. Since the ureas from DIC and
CIC were relatively soluble in CH2Cl2, these reagents were more
suitable for solid-phase peptide synthesis than DCC. The solubilities
of N-cyclohexyl-N0-isopropylurea, N,N0-diisopropylurea, and N,N0-dicyclohexylurea
were 30, 5.2 and 1.5 g/L in CH2Cl2, respectively. In Fmoc solid-phase
peptide synthesis, the DIC/additive method was investigated in various
conditions by changing the additive, base,and solvent. Carpino demonstrated
that DIC/HOAt was superior to DIC/other additives.43 Further variations
of the carbodiimide such as BMC, BEC, and N,N0-dicyclopentylcarbodiimide
were reported (Fig. 12).44 Rapoport developed the hydrophilic side-chain-containing
carbodiimide, BDDC, in 1994. BDDC in THF, DMF, or toluene gave
a reasonable yield for the coupling reaction with a Bocprotected
amino acid and the by-product was easily removed by an acid wash.
http://www.peptide-and-dna.com/
The
formation of the amide bond together with chirality of the molecules
plays a key role in the preparation of a broad range of organic
compounds. It begins with the small molecules through the peptides
to fully synthetic proteins. Today, the synthesis of peptide or
protein based pharmaceutical drug requires up to 100 steps, the
name of the game is ��production cost�� and the modern synthetic
technologies play a central role in this battleA. thorough study
of the mechanism in each method involving basic producer for logistic
and technical support is a necessary term for the optimization of
the coupling step of a new peptide drug and further up-scaling towards
bulk manufacturing. Today, Cl-HOBt as an additive and HCTU/TCTU
are excellent alternatives to the most classic methods for the large
scale manufacturing of peptide and proteins.
Local:
Carbodiimides
are a group of organic compounds which have the resonance formula
N=C=N. Carbodiimide is formed by dehydration of urea or from thiourea.
Carbodiimides are readily reacts with various form of amines and
hydroxyl functional
groups. Carbodiimides are used as dehydration agents and
as activating agent of carboxylic acids to form esters or amides. The disubstituted
carboxyl activating agents are used for crosslinking
proteins to nucleic acids and formation of immunoconjugates
in peptide synthesis. N,N'-dicyclohexylcarbodiimide (DCC)
is one of the most
common peptide coupling agents. It is a low melting point waxy solid; insoluble
in water but highly soluble in common organic solvents
like acetonitrile dichloromethane, dimethylformamide,
and tetrahydrofuran. DCC is a potent allergen. N,N'-diisopropylcarbodiimide
(DIC) is an alternative to DCC, DIC is a liquid and
is not an allergen. 1-Ethyl-3-(3-dimethylaminopropyl)carbodiimide
(EDC) is a water-soluble
activating agent for amide bonding with primary amines. It also activates phosphate
groups. Typically, it is utilized in the pH
range 4.0-6.0 without buffers. In particular, amine and carboxylate buffers
should be avoided. Carbodiimides
are so active and cause racemization of the
amino acid. Active esters are
less reactive and less in danger of racemization. 1-Hydroxybenzotriazole (HOBt) and
1-hydroxy-7-aza-benzotriazole (HOAt)
are substances that react with the O-acylurea to form active esters.
HOAt is a condensation
additive in peptide synthesis. It
efficiently speeds up coupling process,
reduces the loss of chiral integrity, and
provides a visual indication (yellow to
colorless) of the reaction course. Other
active esters exit as non-nucleophilic anionic salts
of uronium or phosphonium
such as O-(Benzotriazol-1-yl)-N,N,N',N'-tetramethyluronium
hexafluorophosphate(HBTU), O-(7-Azabenzotriazol-1-yl)-N,N,N',N'-tetramethyluronium
hexafluorophosphate (HATU), (Benzotriazol-1-yloxy)tripyrrolidinophosphonium hexafluorophosphate
(PyBOP). Carbodiimides
are a group of organic compounds which have the resonance formula
N=C=N having .Carbodiimides are formed by dehydration of ureas or from thioureas.
Carbodiimides are readily reacts with various form of amines and
hydroxyl functional
groups. Carbodiimides are used as dehydration agents and
as activating agent of carboxylic acids to form esters or amides. The disubstituted
carboxyl activating agents are used for crosslinking
proteins to nucleic acids and formation of immunoconjugates
in peptide synthesis. N,N'-dicyclohexylcarbodiimide (DCC)
is one of the most
common peptide coupling agents. It is a low melting point waxy solid; insoluble
in water but highly soluble in common organic solvents
like acetonitrile dichloromethane, dimethylformamide,
and tetrahydrofuran. DCC is a potent allergen. N,N'-diisopropylcarbodiimide
(DIC) is an alternative to DCC, DIC is a liquid and
is not an allergen. 1-Ethyl-3-(3-dimethylaminopropyl)carbodiimide
(EDC) is a water-soluble
activating agent for amide bonding with primary amines. It also activates phosphate
groups. Typically, it is utilized in the pH
range 4.0-6.0 without buffers. In particular, amine and carboxylate buffers
should be avoided. Carbodiimides
are so active and cause racemization of the
amino acid. Active esters are
less reactive and less in danger of racemization. 1-Hydroxybenzotriazole (HOBt) and
1-hydroxy-7-aza-benzotriazole (HOAt)
are substances that react with the O-acylurea to form active esters.
HOAt is a condensation
additive in peptide synthesis. It
efficiently speeds up coupling process,
reduces the loss of chiral integrity, and
provides a visual indication (yellow to
colorless) of the reaction course. Other
active esters exit as non-nucleophilic anionic salts
of uronium or phosphonium
such as O-(Benzotriazol-1-yl)-N,N,N',N'-tetramethyluronium
hexafluorophosphate(HBTU), O-(7-Azabenzotriazol-1-yl)-N,N,N',N'-tetramethyluronium
hexafluorophosphate (HATU), (Benzotriazol-1-yloxy)tripyrrolidinophosphonium hexafluorophosphate
(PyBOP).
CARBODIIMIDES
|
Product |
CAS RN |
|
N,N'-Dicyclohexylcarbodiimide |
538-75-0 |
| N,N'-Diphenylcarbodiimide | 622-16-2 |
|
N,N'-Di-tert-butylcarbodiimide |
691-24-7 |
|
N,N'-Diisopropylcarbodiimide |
693-13-0 |
|
1,3-Di-p-tolylcarbodiimide |
726-42-1 |
| Bis(3-chloro-2-methylphenyl)carbodiimide | 961-63-7 |
| Bis(trimethylsilyl)carbodiimide | 1000-70-0 |
| Bis(o-tolylcarbodiimide) | 1215-57-2 |
|
1-tert-Butyl-3-ethylcarbodiimide |
1433-27-8 |
|
N-(3-Dimethylaminopropyl)-N'-ethylcarbodiimide |
1892-57-5 |
| Bis(2,6-diisopropylphenyl)carbodiimide | 2162-74-5 |
| Bis(2,6-diethylphenyl)carbodiimide | 2162-75-6 |
|
N-Cyclohexyl-N'-(2-morpholinoethyl)carbodiimide metho-p-toluenesulfonate |
2491-17-0 |
| N-Cyclohexyl-N'-isopropylcarbodiimide | 3496-83-1 |
| N-Methyl-N'-phenylcarbodiimide | 4172-91-2 |
| 1-Cyclohexyl-3-(2-(4-morpholinyl)ethyl)carbodiimide | 15580-20-8 |
|
1-(3-(Dimethylamino)propyl)-3-ethylcarbodiimide methiodide |
22572-40-3 |
| N,N'-Dicyclohexyl-N-methylcarbodiimidium iodide | 36049-77-1 |
| N-(2,2,6,6-Tetramethylpiperidyl-1-oxyl) N'-(cyclohexyl)carbodiimide | 42249-40-1 |
| 1-Ethyl-3-(4-azonia-4,4-dimethylpentyl)carbodiimide | 45024-77-9 |
| Bis(2,4,6-triisopropyl-3-isocyanatophenyl)carbodiimide | 68083-39-6 |
| 1-Phenyl-3-trimethylaminopropyl carbodiimide | 79322-24-0 |
| N-Cyclohexyl-N'-(4-dimethylamino-alpha-naphthyl)carbodiimide | 86332-16-3 |
| N-Cyclohexyl-N'-(1-pyrenyl)carbodiimide | 98540-87-5 |
| 1-Ethyl-3-(3-dimethylaminoethyl)carbodiimide | 141650-20-6 |
APPEARANCE
ASSAY
HAZARD OVERVIEW
OSHA Hazards:Target Organ Effect, Highly toxic by inhalation, Harmful by ingestion., Highly toxic by skin absorption, Skin sensitiser, Corrosive. Target Organs:Eyes
GHS
PICTOGRAMS


HAZARD STATEMENTS
H302-H311-H317-H318
P STATEMENTS
P280-P305 + P351 + P338-P312
![]() T+
Very toxic
T+
Very toxic
RISK PHRASES
22-24-41-43
SAFETY PHRASES
24-26-37/39-45