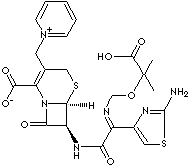
PRODUCT IDENTIFICATION
78439-06-2 (Pentahydrate)
H.S. CODE
TOXICITY
CLASSIFICATION
EXTRA NOTES
Contains sodium carbonate (10%) as stabilizer
PHYSICAL AND CHEMICAL PROPERTIES
AUTOIGNITION
NFPA RATINGS
Health hazard: 2, Fire: 0, Reactivity Hazard: 0
REFRACTIVE INDEX
EXTERNAL LINKS & GENERAL DESCRIPTION
Drug Information Portal (U.S. National Library of Medicine) - Ceftazidime
PubChem Compound Summary - Ceftazidime
http://www.drugbank.ca
Ceftazidime is a semisynthetic, broad-spectrum, beta-lactam antibiotic for
parenteral administration. Ceftazidime is bactericidal in action exerting its
effect by inhibition of enzymes responsible for cell-wall synthesis, primarily
penicillin binding protein 3 (PBP3). A wide range of gram-negative organisms is
susceptible to ceftazidime in vitro, including strains resistant to gentamicin
and other aminoglycosides. In addition, ceftazidime has been shown to be active
against gram-positive organisms. It is highly stable to most clinically
important beta-lactamases, plasmid or chromosomal, which are produced by both
gram-negative and gram-positive organisms and, consequently, is active against
many strains resistant to ampicillin and other cephalosporins. Ceftazidime has
activity against the gram-negative organisms Pseudomonas and
Enterobacteriaceae. Its activity against Pseudomonas is a
distinguishing feature of ceftazidime among the cephalosporins.
Local:
Cephalosporin: any of a group of broad-spectrum derived from species of fungi of
the genus Cephalosporium and are related to the penicillins in both structure
and mode of action but relatively penicillinase-resistant antibiotics. These
antibiotics have low toxicity for the host, considering their broad
antibacterial spectrum. They have the active nucleus of beta-lactam ring which
results in a variety of antibacterial and pharmacologic characteristics when
modified mainly by substitution at 3 and 7 positions. Their antibacterial activities
result from the inhibition of mucopeptide synthesis in the cell wall. They are
widely used to treat gonorrhea, meningitis, pneumococcal, staphylococcal and
streptococcal infections. The cephalosporin class of antibiotics is usually
divided into generations by their antimicrobial properties. Three generations of
cephalosporins are recognized and the fourth has been grouped. Each newer
generation of cephalosporins has broader range of activity against gram-negative
organisms but a narrower range of activity against gram-positive organisms than
the preceding generation. The newer agents have much longer half-lives resulting
in the decrease of dosing frequency. Accordingly, the third-generation
cephalosporins can penetrate into tissues well, and thus antibiotic levels are
good in various body fluids. Third-generation cephalosporins are more active
against gram-negative organisms but less active against gram-positive organisms
than second-generation agents; examples are cefoperazone, cefotaxime,
ceftriaxone, ceftazidime, ceftizoxime, and moxalactam.
APPEARANCE
ASSAY
pH
HEAVY METALS
20ppm max
INDIVIDUAL IMPURITY
Hazard OVERVIEW
Irritant
GHS
PICTOGRAMS

HAZARD STATEMENTS
H317 May cause
an allergic skin reaction
H319 Causes serious eye irritation
H334
May cause allergy or asthma symptoms or breathing difficulties
if inhaled
PRECAUTIONARY STATEMENTS
P261
Avoid breathing dust/fume/gas/mist/vapours/spray.
P280
Wear protective gloves/protective clothing/eye protection/face
protection.
P305 + P351 + P338 IF IN EYES: Rinse cautiously
with water for several minutes. Remove contact lenses, if present
and easy to do. Continue rinsing.
P342 + P311 IF experiencing
respiratory symptoms: call a POISON CENTER or doctor/physician.
![]() Xn
Harmful
Xn
Harmful
RISK PHRASES
SAFETY PHRASES
22 Do not breathe dust
36/37
Wear suitable protective clothing and gloves
45 In case of accident or if you feel unwell, seek medical
advice immediately (show label where possible)