PRODUCT IDENTIFICATION
H.S. CODE
TOXICITY
CLASSIFICATION
PHYSICAL AND CHEMICAL PROPERTIES
AUTOIGNITION
NFPA RATINGS
Health hazard 0, Fire: 1, Reactivity Hazard 0
REFRACTIVE INDEX
Stable under ordinary conditions
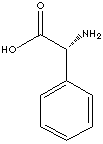
GENERAL DESCRIPTION & APPLICATIONS
In chemistry, a side chain also often called R-group is a stable and covalently linked part attached to a core structure to modify and to gain different properties. Some examples of Cephalosporin side chains include:
http://aac.asm.org/
Structure-Activity
Relationship of Carbacephalosporins and Cephalosporins: Antibacterial
Activity and Interaction with the Intestinal Proton-Dependent Dipeptide
Transport Carrier of Caco-2 Cells.
http://dissertations.ub.rug.nl/
....The substitution of the side chain of penG, phenyl acetic acid, with other
side chains like D-phenylglycine and D-p-hydroxyphenylglycine, resulted in the
semi-synthetic ��-lactam antibiotics ampicillin and amoxicillin (Figure 1),
respectively, which are more stable and can be orally administered. Substitutions
at C2 of the penicillin core resulted in new ��-lactam antibiotics to which
resistant bacteria are sensitive.....
http://dissertations.ub.rug.nl/
A semi-random mutagenesis approach was followed to increase the
performance of penicillin acylase PAS2 in the kinetically controlled synthesis
of ampicillin from 6-aminopenicillanic acid (6-APA) and activated D-phenylglycine
derivatives. We directed changes in amino acid residues to positions close
to the active site that are expected to affect the catalytic performance of penicillin
acylase: aR160, aF161, and bF24. From the resulting triple mutant gene bank,
improved PAS2 mutants were recovered by only screening 700 active mutants with a HPLC-based screening method. A detailed kinetic analysis of the three
most promising mutants, T23, TM33, and TM38, is presented. These mutants
allowed the accumulation of ampicillin at 4 to 5 times higher concentrations
than the wild-type enzyme, using D-phenylglycine methyl ester as the acyl donor.
At the same time, the loss of activated acyl donor due to the competitive hydrolytic
side reactions could be reduced to less than 20 % with the mutant enzymes
compared to > 80 % with wild-type PAS2. Although catalytic activity dropped
by a factor 5 to 10, the enhanced synthetic performance of the recovered penicillin
acylase variants makes them interesting biocatalysts for the production of
blactam antibiotics.
http://etds.ncl.edu.tw/
D-p-hydroxyphenylglycine (D-pHPG) is a D-amino acid which can be used to prepare
semisynthetic antibiotics Amoxicillin. D-pHPG can be produced by two enzymatic
steps. The substrate D-p-hydroxyphenylhydantoin (pHPH) is stereo-specifically
hydrolysed by D-hydantoinase to the
N-carbamoyl-D-p-hydroxyphenylglycine(D-CpHPG). CpHPG can be further converted to
D-pHPG by a second enzymatic step catalysed by an N-carbamyl-amino acid
amidohydrolase.The bacterium Agrobacterium radiobacter can produce both
D-hydantoinase and the N-carbamoyl-amino-acid amidohydrolase. The aim of this
thesis is to develope an optimized complex medium for A. radiobacter and to
evaluate pHPH and soy bean hydrolysate as inducers for the both enzymes.
Fed-batch fermentation using a pre-determined feeding profiles was carried out
for high cell density fermentation of A. radiobacter . The higest cell
concentration obtained was 19.48g DCW/L with specific activity of 0.279
µmole/hr.mg cell . In order to reuse the D-hydantoinase and
amidohydrolase of the A. radiobacter cell a simple cell immobilization method
was proposed. The cells suspension was floculated with PEI and further
cross-linked with glutaraldehyde and chitosan After ten repeated batch reaction
,the immobilyed cell still can convert 4% (w/v)pHPH into D-pHPG with 58.5%
conversion.
APPEARANCE
ASSAY
99.0% min
SPECIFIC ROTATION
-161° ~ -155°
HEAVY METALS
20ppm max
WATER
0.2% max
HAZARD OVERVIEW
GHS (Globally Harmonised System) Classification: Not a dangerous substance. Potential Health Effects: Eyes - May cause eye irritation. Skin - May be harmful if absorbed through skin May cause skin irritation. Inhalation - May be harmful if inhaled. May cause respiratory tract irritation. Ingestion - May be harmful if swallowed.
RISK PHRASES
36/37/38
SAFETY PHRASES
26-36