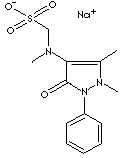
PRODUCT IDENTIFICATION
H.S. CODE
TOXICITY
CLASSIFICATION
EXTRA NOTES
PHYSICAL AND CHEMICAL PROPERTIES
SOLVENT SOLUBILITY
REFRACTIVE INDEX
Stable under ordinary conditions
EXTERNAL LINKS &GENERAL DESCRIPTION
http://www.clinchem.org/
Dipyrone (metamizole) is widely used and has effective analgesic, antipyretic,
and antispasmodic properties. After oral or intravenous administration, dipyrone
is rapidly hydrolyzed to the active moiety 4-methylaminoantipyrine (MAA)(8)(9).
MAA is further metabolized to 4-formylaminoantipyrine (FAA) and
4-aminoantipyrine (AA), which is acetylated to 4-acetylaminoantipyrine (AAA).
These 4 major metabolites account for 60% of the administered dose excreted in
urine. To determine whether dipyrone and/or its metabolites interfere with
the measurement of plasma catecholamines, we prepared solutions of dipyrone (1
mg/mL) and MAA, AA, FAA, and AAA (50 µg/mL) corresponding to 5 times the
expected maximum concentration obtained after a 1-g dose of dipyrone(9).
Dipyrone caused a peak at the expected retention time of dopamine (Fig. 1C ) and
another broad peak in the next chromatogram at the retention time of DHBA when
an aqueous injection was started after 25 min (not shown). The bioactive
metabolite MAA also had a retention time of 13 min in the next analysis, and
thus it interfered with the internal standard (Fig. 1D ). These findings
indicate that the interfering peak is MAA, which is readily formed from the
chemically labile drug dipyrone(9). AA eluted after 23 min, and no signal was
observed for FAA or AAA.
http://cpj.sagepub.com/
This study compared the antipyretic effectiveness of acetaminophen, ibuprofen,
and dipyrone in young children with fever. The results were based on a modified
double-blind, randomized, multinational trial that evaluated 628 febrile
children, aged 6 months to 6 year. All three drugs lowered temperature in the
555 patients completing the study. Temperature normalization rates in the
ibuprofen and dipyrone groups (78% and 82%, respectively) were significantly
higher than the acetaminophen group (68%, P=0.004). After 4 to 6 hours, mean
temperature in the dipyrone group was significantly lower than the other groups,
demonstrating longer temperature normalization with dipyrone. All three drugs
showed comparable tolerability profiles.
http://www.drugs.com/
Dipyrone is not approved for marketing in the United States by the US Food and
Drug Administration or in Canada and many European countries because of its
adverse reactions, including agranulocytosis. However, it is widely used in
other countries during labor and breastfeeding.[1][2][3][4] After ingestion by
the mother, dipyrone and its metabolites appear in breastmilk in rather large
amounts. It is found in the blood and urine of breastfed infants and can cause
pharmacological effects in the breastfed infant. One case of cyanotic episodes
in a breastfed infant was attributed to dipyrone in breastmilk. The drug and
metabolites are eliminated from the breastmilk by 48 hours after a dose and one
manufacturer recommends no breastfeeding for 48 hours after a dose.[5] Safer
alternatives are available for analgesia during breastfeeding.
APPEARANCE
ASSAY
99.0% min
MOISTURE
0.18% max
RESIDUE ON IGNITION
0.2% max
HEAVY METALS
10ppm max
As
1.5ppm max