PRODUCT IDENTIFICATION
H.S. CODE
TOXICITY
CLASSIFICATION
PHYSICAL AND CHEMICAL PROPERTIES
REFRACTIVE INDEX
GENERAL DESCRIPTION & APPLICATIONS
APPEARANCE
98.0% min
265 - 270 C
SULFATED ASH
0.2% max
LOSS ON DRYING
0.5% max
|
|
|
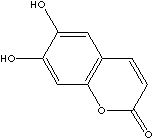
| ESCULETIN | ||
|
PRODUCT IDENTIFICATION |
||
| CAS NO. | 305-01-1 |
|
| EINECS NO. | 206-161-5 | |
| FORMULA | C9H6O4 | |
| MOL WT. | 178.14 | |
|
H.S. CODE |
||
|
TOXICITY |
||
| SYNONYMS | 6,7-Dihydroxycoumarin; Cichorigenin; Esculetol; | |
| 6,7-Dihydroxy-2-benzopyrone; 6,7-Dihidroxi-2-benzopirona; 6,7-Dihydroxy-2-benzopyrone; | ||
| SMILES |
|
|
|
CLASSIFICATION |
||
|
PHYSICAL AND CHEMICAL PROPERTIES |
||
| PHYSICAL STATE | yellow crystalline powder | |
| MELTING POINT | 265 - 270 C | |
| BOILING POINT |
|
|
| SPECIFIC GRAVITY |
|
|
| SOLUBILITY IN WATER | Slightly soluble | |
| pH | weak acidic | |
| VAPOR DENSITY |
|
|
| AUTOIGNITION | ||
|
REFRACTIVE INDEX |
|
|
| NFPA RATINGS | Health: 1; Flammability: 0; Reactivity: 0 | |
| FLASH POINT |
|
|
| STABILITY | Stable under normal conditions. | |
|
GENERAL DESCRIPTION & APPLICATIONS |
||
| Coumarin (anhydride of o-coumaric acid) is white, crystalline lactone, obtainable naturally from several plants, such as tonka bean, lavender, sweet clover grass, strawberries, and cinnamon, or produced synthetically from an amino acid, phenylalanine. It is the principle of dicumarol which inhibits the hepatic synthesis of the vitamin K–dependent coagulation factors. Coumarin derivatives are used widely as anticoagulants (such as warfarin, -OH group is attached at 4 position) for the treatment of disorders in which there is excessive or undesirable clotting, such as thrombophlebitis, pulmonary embolism, and certain cardiac conditions. Coumarin derivatives are also used as rodenticides due to the property of causing fatal hemorrhaging. Coumarin has the characteristic odour like that of vanilla beans. It is used for the preparation of perfumes, soaps, flavourings. The coumarin nucleus (benzo-2-pyrone) is derived cinnamic acid (phenylacrylic skeleton) in the biosynthesis. Accordingly, the hydroxy group attached to coumarin structure at 7 position is important in biosynthesis pathway. Umbelliferone (7-hydroxy coumarin), esculetin (6,7-Dihydroxycoumarin), scopoletin (7-hydroxy-6-methoxycoumarin) are the widespread coumarins in nature. Synthetic 7-hydroxy coumarins are used to absorb ultraviolet rays in sunscreen cosmetics and used in the synthesis of drugs especially anticancer. Scopoletin is used a peroxide or calcium signal during the oxidative burst. It is a acetylcholinesterase inhibitor. | ||
| SALES SPECIFICATION | ||
|
APPEARANCE |
yellow crystalline powder | |
| ASSAY |
98.0% min |
|
| MELTING POINT |
265 - 270 C |
|
|
SULFATED ASH |
0.2% max |
|
|
LOSS ON DRYING |
0.5% max |
|
| TRANSPORTATION | ||
| PACKING |
|
|
| HAZARD CLASS | ||
| UN NO. | ||
| OTHER INFORMATION | ||
| Hazard Symbols: T, Risk Phrases: 23/24/25, Safety Phrases: 26-36 | ||
|
|
|