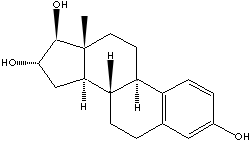
| ESTRIOL | ||
|
PRODUCT IDENTIFICATION |
||
| CAS NO. | 50-27-1 |
|
| EINECS NO. | 200-022-2 | |
| FORMULA | C18H24O3 | |
| MOL WT. | 288.39 | |
|
H.S. CODE |
2937.92.0000 | |
|
TOXICITY |
Rat LD50 (Oral):> 2gm/kg | |
| SYNONYMS | Drihydroxyestrin; Oestriol; | |
|
(16alpha,17beta)-Estra-1,3,5(10)-triene-3,16,17-triol; (16alpha,17beta)-Oestra-1,3,5(10) -triene-3,16,17-triol; 1,3,5(10)-Estratriene-3,16alpha,17beta-triol; 16alpha-Hydroxyestradiol; 3,16alpha,17beta-Trihydroxy-1,3,5(10)-estratriene; 1,3,5-Estratriene-3beta,16alpha,17beta-triol; 1,3,5-Oestratriene-3beta,16alpha,17beta-triol; 16-alpha,17-beta-Estriol; 16-alpha,17-beta-Oestriol; 16-alpha-Hydroxyestradiol; Destriol; 16-alpha-Hydroxyoestradiol; 16alpha,17beta-Estriol; 16alpha,17beta-Oestriol; 16alpha-Hydroxy-17beta-estradiol; 16alpha-Hydroxyestradiol; 16alpha-Hydroxyoestradiol; 3,16-alpha,17-beta-Estriol; 3,16-alpha,17-beta-Oestriol; 3,16-alpha,17-beta- Trihydroxy-delta-1,3,5- estratriene; 3,16-alpha,17-beta-Trihydroxy-delta-1,3,5 -oestratriene; 3,16-alpha,17-beta-Trihydroxyestra- 1,3,5(10)-triene; 3,16-alpha,17-beta-Trihydroxyoestra -1,3,5(10)-triene; 3,16alpha,17beta-Estriol; 3,16alpha,17beta-Trihydroxy-1,3,5(10)-estratriene; 3,16alpha,17beta-Trihydroxy-delta-1,3,5 -oestratriene; Estra-1,3,5(10)-trien-3,16alpha,17beta-triol; Estra-1,3,5(10)-triene-3,16alpha, 17beta-triol; Estratriol; Estriolo; Follicular hormone hydrate; Oestra-1,3,5(10)-triene-3,16-alpha,17 -beta-triol; Oestra-1,3,5(10)- triene-3,16alpha,17beta-triol; Oestratriol; Oestriol; Oestriolum; Orestin; Orgastyptin; Thulol; Tridestrin; Trihydroxyestrin; Trihydroxyoestrin; Triodurin; Triovex; Delta(1,3,5-10)-estratriene-3,16-cis-17-trans-diol; ��߲��; |
||
| SMILES |
[C@H]12[C@H]3[C@@H](c4c(CC3)cc(cc4)O)CC[C@@]1 ([C@H]([C@@H](C2)O)O)C |
|
|
CLASSIFICATION |
Steroid, Estrogen, Hormone, Biochemicals ex Plants |
|
|
PHYSICAL AND CHEMICAL PROPERTIES |
||
| PHYSICAL STATE |
white to almost white crystalline powder |
|
| MELTING POINT | 284 - 285 C | |
| BOILING POINT | ||
| SPECIFIC GRAVITY |
|
|
| SOLUBILITY IN WATER | practically insoluble (441mg/l at 25 C) | |
|
SOLVENT SOLUBILITY |
sparingly soluble in alcohol) | |
| VAPOR DENSITY |
|
|
| pKa | (Dissociation Constant at 20 C) | |
| log Pow | 2.45 (Octanol-water) | |
| VAPOR PRESSURE | 1.97E-10 (mmHg at 25 C) | |
| HENRY'S LAW | 1.33E-12 (atm-m3/mole at 25 C) | |
| OH RATE | 1.29E-10 (cm3/molecule-sec at 25 C Atmospheric) | |
| AUTOIGNITION |
|
|
| NFPA RATINGS | ||
|
REFRACTIVE INDEX |
||
| FLASH POINT |
|
|
| STABILITY |
|
|
|
GENERAL DESCRIPTION & EXTERNAL LINKS |
||
| A hydroxylated metabolite of 17beta-estradiol or estrone that has a hydroxyl group at C3-beta, 16-alpha, and 17-beta position. Estriol is a major urinary estrogen.
Large amount of estriol and estrone are produced by the placenta during pregnancy. These are also the primary estrogens
produced by adipose tissue in men and in post-menopausal women. Isomers
with inversion of the hydroxyl group or groups are called epiestriol. Female
hormone replacement. Three major estrogens
possessing the biologic activity of estrus-producing
hormones are estradiol, estrone and
estriol.
Wikipedia Linking: http://en.wikipedia.org/wiki/Estriol http://www.reciprocalnet.org/ http://clinicaltrials.gov/show/NCT00908570 |
||
| SALES SPECIFICATION | ||
|
APPEARANCE |
white to almost white crystalline powder |
|
|
ASSAY |
97.0-103.0% (on dry basis) | |
|
RESIDUES ON IGNITION |
0.1% max |
|
|
LOSS ON DRYING |
0.5% max |
|
|
HEAVY METALS |
10ppm max |
|
|
SPECIFIC ROTATION |
+54° ~ +62° |
|
|
PARTICLE SIZE |
20 ��m |
|
| TRANSPORTATION | ||
| PACKING |
|
|
| HAZARD CLASS | ||
| UN NO. | ||
| OTHER INFORMATION | ||
|
Hazard Symbols:T, Risk Phrases:60-61-40, Safety Phrases:53-22-36/37/39-45 |
||