PRODUCT IDENTIFICATION
108885-67-2 (HCl)
H.S. CODE
TOXICITY
CLASSIFICATION
Antagonist, Anti-ulcer agent, Gastrointestinal agent, Histamine agent, Histamine H2 antagonist, Guanidine, Thiazole, Thioether, Sulfonamide
EXTRA NOTES
ATC code: A02BA03
PHYSICAL AND CHEMICAL PROPERTIES
REFRACTIVE INDEX
Stable under ordinary conditions.
EXTERNAL LINKS & GENERAL DESCRIPTION
Drug Information Portal (U.S. National Library of Medicine) - Famotidine
http://www.drugbank.ca/
Mechanism
of action: Famotidine binds competitively to H2-receptors located
on the basolateral membrane of the parietal cell, blocking histamine
affects. This competitive inhibition results in reduced basal and
nocturnal gastric acid secretion and a reduction in gastric volume,
acidity, and amount of gastric acid released in response to stimuli
including food, caffeine, insulin, betazole, or pentagastrin.
http://www.egeneralmedical.com/
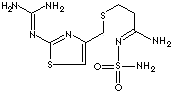
The active ingredient in Pepcid is a histamine H2-receptor
antagonist. Famotidine is N'-(aminosulfonyl)-3-[[[2-[(diaminomethylene)amino]-4-thiazolyl]methyl]thio]
propanimidamide. The empirical
formula of famotidine is
C8H15N7O2S3 and its
molecular weight is 337.43. Famotidine is a white to pale yellow crystalline compound that is freely
soluble in glacial acetic acid, slightly soluble in methanol, very slightly
soluble in water, and practically insoluble in ethanol. Each tablet for oral administration contains either 20 mg or 40 mg of
famotidine. Inactive Ingredients: Hydroxypropyl cellulose, hydroxypropyl
methylcellulose, iron oxides, magnesium stearate, microcrystalline cellulose,
starch, talc, titanium dioxide. Each Pepcid RPD orally disintegrating tablet for oral administration contains
either 20 mg or 40 mg of famotidine and the following inactive ingredients:
aspartame, mint flavor, gelatin, mannitol, red ferric oxide, and xanthan
gum. Each 5 ml of the oral suspension when prepared as directed contains 40 mg of
famotidine and the following inactive ingredients: citric acid, flavors,
microcrystalline cellulose and carboxymethylcellulose sodium, sucrose and
xanthan gum. Added as preservatives are sodium benzoate 0.1%, sodium
methylparaben 0.1%, and sodium propylparaben 0.02%. Each ml of the solution for intravenous injection contains 10 mg of
famotidine. Inactive Ingredients: L-aspartic acid 4 mg, mannitol 20 mg,
and Water for Injection q.s. 1 ml. The multidose injection also contains benzyl
alcohol 0.9% added as preservative.
Local:
Famotidine is a histamine H2-receptor antagonist (also called H2-blocker) which
decreases the amount of acid produced by the stomach and is used to treat
gastric and duodenal ulcers by blocking the H2 subtype of histamine receptors.
Examples of H2-blockers are cimetidine, famotidine and nizatidine. The chemical
designation of famotidine is N'-(aminosulfonyl)-3-[[[2-
[(diaminomethylene) amino]- 4-thiazolyl] methyl]thio] propanimidamide. It is a white
to pale yellow crystalline compound; very slightly soluble in water, insoluble
in alcohol, chloroform (slightly soluble in methanol), freely soluble in glacial
acetic and DMF; melting point 161 - 163 C. Examples of H2-receptor antagonist
include
|
|
APPEARANCE
IDENTIFICATION
passes test A,B
98.0.% - 101.0%
RELATED SUBSTANCE
Complies
RESIDUE ON IGNITION
0.1% max
VOLATILES
Complies
HEAVY METALS
10ppm max