PRODUCT IDENTIFICATION
H.S. CODE
TOXICITY
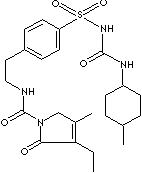
1-[[p-[2-(3-ethyl-4-methyl-2-oxo-3-pyrroline-1-carboxamido) ethyl]phenyl] sulfonyl]-3-(trans-4- methyl cyclohexyl) Urea; trans-2,5-Dihydro-3-ethyl-4-methyl-N-(2-(4-(((((4-methylcyclohexyl)amino) carbonyl)amino)sulfonyl)phenyl)ethyl)-2-oxo-1H-pyrrole-1-carboxamide; Endial;
CLASSIFICATION
PHYSICAL AND CHEMICAL PROPERTIES
AUTOIGNITION
NFPA RATINGS
REFRACTIVE INDEX
GENERAL DESCRIPTION & APPLICATIONS
Glimepiride, belongs to the class of sulfonylurea, is an oral blood-glucose-lowering drug actong for medium- to-long period. It is a white to yellowish crystalline powder with odorless; melting point 205 -207 C. The chemical designation is 1-[[p-[2-(3-ethyl-4-methyl-2-oxo-3-pyrroline-1-carboxamido) ethyl]phenyl] sulfonyl]-3-(trans-4-methylcyclohexyl) Urea.
Some classes of drugs of anti-diabete include:
- Sulfonylureas
- Chlorpropamide (CAS#: 94-20-2)
- Tolbutamide (CAS#: 64-77-7)
- Tolazamide (CAS#: 1156-19-0)
- Glipizide (CAS#: 29094-61-9)
- Glyburide (CAS#: 10238-21-8)
- Gliclazide (CAS#: 21187-98-4)
- Glibenclamide (CAS#: 10238-21-8)
- Glimepiride (CAS#: 93479-97-1)
- Gliquidone (CAS#: 33342-05-1)
- Meglitinide analogues
- Repaglinide (CAS#: 135062-02-1)
- Nateglinide (CAS#: 105816-04-4)
- Meglitinide (CAS#: 54870-28-9)
- Mitiglinide (CAS#: 145375-43-5)
- Mitiglinide Calcium (CAS #: 145525-41-3)
- Biguanide
- Metformin (CAS#: 657-24-9)
- Phenformin (CAS#: 114-86-3)
- Thiazolidinediones (Glitazones)
- Rosiglitazone (CAS#: 155141-29-0)
- Pioglitazone (CAS#: 111025-46-8)
- Alpha-glucosidase Inhibitors
- Acarbose (CAS#: 56180-94-0)
- Miglitol (CAS#: 72432-03-2)
- Dipeptidylpeptidase IV inhibitor
- Saxagliptin (CAS#: 361442-04-8)
- Sitagliptin (CAS#: 790712-60-6)
- Vildagliptin (CAS#: 274901-16-5)
APPEARANCE
ASSAY (HPLC)
MELTING POINT
205 - 207 C
LOSS ON DRYING
0.5% max
TOTAL IMPURITY
1.0% max
20ppm max