| CAS
NO. |
27885-92-3 |
|
| EINECS NO. |
248-711-7 |
| FORMULA |
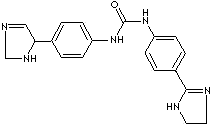
C19H20N6O |
| MOL
WT. |
348.41 |
|
H.S.
CODE
|
|
|
TOXICITY
|
|
| SYNONYMS |
Imizol; 1,3-Bis(3-(2-imidazolin-2-yl)phenyl)urea;
|
|
N,N'-Bis[3-(4,5-dihydro-1H-imidazol-2-yl)-phenyl]
Urea; 3,3'-di-2-imidazolin-2-yl-carbanilide; |
|
SMILES |
|
|
CLASSIFICATION
|
|
|
PHYSICAL AND CHEMICAL PROPERTIES
|
| PHYSICAL
STATE |
White crystalline
powder |
| MELTING POINT |
350
C |
| BOILING
POINT |
|
| SPECIFIC GRAVITY |
|
| SOLUBILITY
IN WATER |
|
| pH |
|
| VAPOR DENSITY |
|
|
AUTOIGNITION
|
|
|
NFPA
RATINGS
|
|
|
REFRACTIVE
INDEX
|
|
| FLASH
POINT |
|
| STABILITY |
Stable
under ordinary conditions. |
|
APPLICATIONS
|
Imidocarb is a veterinary drug used as an antiprotozoal agent against
Babesia. Chemical designation is
N,N'-Bis[3-(4,5-dihydro-1H-imidazol-2-yl)-phenyl] urea. It is white powder;
melting point 350 C. The dipropionate or hydrochloride salts are for
intramuscular or subcutaneous administration.
| Imidocarb hydrochloride | CAS
RN: 5318-76-3 |
| Imidocarb dipropionate | CAS
RN: 55750-06-6 |
|
| SALES
SPECIFICATION |
|
APPEARANCE
|
White crystalline
powder |
|
IDENTIFICATION
|
Complies
|
|
ASSAY
(HPLC) |
98.5%
min |
|
LOSS
ON DRYING
|
1.0%
max
|
|
HEAVY METALS |
10ppm
max
|
|
SULFATED ASH |
0.1% max |
| TRANSPORTATION |
| PACKING |
|
| HAZARD CLASS |
|
| UN
NO. |
|
| OTHER
INFORMATION |
|
|
