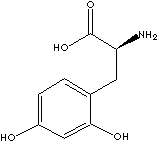
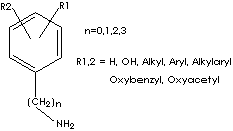| CAS
NO. |
59-92-7 |
|
| EINECS NO. |
200-445-2 |
| FORMULA |
(HO)2C6H3CH2CH(NH2)COOH |
| MOL
WT. |
197.19 |
|
H.S.
CODE
|
|
|
TOXICITY
|
|
| SYNONYMS |
3,4-Dihydroxy-L-phenylalanine; |
| [3-(3,4-Dihydroxyphenyl)-L-Alanine];
L-3-(3,4-Dihydroxyphenyl)alanine; 3-hydroxy-L-tyrosine;
L-3,4-dihydroxyphenylalanine;
L-beta-(3,4-Dihydroxyphenyl)alanine; L-Dihydroxyphenyl-L- alanine; |
| SMILES |
|
|
CLASSIFICATION
|
|
|
PHYSICAL AND CHEMICAL PROPERTIES
|
| PHYSICAL
STATE |
white to
creamy crystalline powder |
| MELTING POINT |
295 C |
| BOILING
POINT |
|
| SPECIFIC GRAVITY |
|
| SOLUBILITY
IN WATER |
Soluble |
|
SOLVENT
SOLUBILITY
|
|
| pH |
|
| VAPOR DENSITY |
|
|
AUTOIGNITION
|
|
|
NFPA
RATINGS
|
|
|
REFRACTIVE
INDEX
|
|
| FLASH
POINT |
|
| STABILITY |
Stable
under ordinary conditions. Air, light sensitive. |
|
APPLICATIONS
|
|
Levodopa, naturally occurring L- form dopa
[(-)-3-(3,4-dihydroxy-phenyl)-L-alanine] is used as an anticholinergic drug and
used in the treatment of Parkinson's disease in which dopamine deficit is seen.
It is used sometimes in combination with carbidopa
[(-)-L-(alpha-hydrazino- (alpha-methyl-beta- (3,4-dihydroxybenzene) propanoic
acid], an inhibitor of aromatic amino acid decarboxylation and entacapone
[(E)-2-cyano-3-(3,4- dihydroxy -5- nitrophenyl)-N,N-diethyl- 2-propenamide], an
inhibitor of catechol-O- methyltransferase. L-dopa is produced by oxidation of
tyrosine by monophenol monooxygenase in the body. It is decarboxylated to
dopamine by the enzyme aromatic-L-amino-acid decarboxylase. It acts as an
intermediate in the biosynthesis of other catecholamines (norepinephrine,
epinephrine), and melanin. It is a white crystalline powder; slightly soluble in
water, in dilute hydrochloric acid and in formic acid; insoluble in ethanol. |
| SALES
SPECIFICATION |
|
BP2000
|
|
APPEARANCE
|
white to
creamy crystalline powder |
|
IDENTIFICATION
|
Complies
|
|
SOLUTION
CLARITY
|
Complies
|
|
pH
|
4.5
- 7.0
|
|
OPTICAL
ROTATION
|
-1.27°
~ -1.34°
|
|
HEAVY
METALS
|
10ppm
max
|
|
SULFATED
ASH
|
0.1%
max
|
|
LOSS
ON DRYING
|
1.0%
max
|
|
CONTENT |
99.0% -
101.0%
|
| TRANSPORTATION |
| PACKING |
|
| HAZARD CLASS |
|
| UN
NO. |
|
| OTHER
INFORMATION |
|
Hazard Symbols: XN, Risk Phrases: 22-36/37/38,
Safety Phrases: 26-36 |
|
GENERAL
DESCRIPTION OF CATECHOLAMINE |
|
Catecholamine: a group of naturally occurring
sympathomimetic amines that have important physiological functions within the
body as neurotransmitters in the central nervous system and hormones in the
blood circulation. Catecholamines are biogenic amines considered as
sympathomimetic drugs; They are characterized by a catechol group [The ortho
(1,2) isomer of dihydroxybenzene] to which is attached an amine group (the
aromatic portion of whose molecule is catechol, and the aliphatic portion an
amine). The most abundant catecholamines in the body are epinephrine
(adrenaline), norepinephrine (noradrenaline) and dopamine. They are derived from
the tyrosine, an amino acid (protein building block) that is the precursor of
norepinephrine. (The prefix nor- describes
normal structure which has no branched chain of carbon atoms. In case of
norepinephrine, it has one less methylene group than its homologue, epinephrine.) Catecholamines belong to a broader class of compounds called
phenethylamines which contain structurally amino acid, phenylalanine and
tyrosine. Phenethylamine is a backbone for the compounds which take roles of
alkaloids as well as hormones and neurotransmitters in nature. Amphetamine is
the substituted phenethylamine by methyl group at alpha position. It is a
synthetic drug used as a diet suppressant and to treat narcolepsy and ADHD
(attention deficit hyperactivity disorder). But amphetamines can produce severe
psychological dependence, including cardiac irregularities and gastric
disturbances. Chronic use often results in extreme exhaustion and malnutrition.
Substituted phenethylamines are:
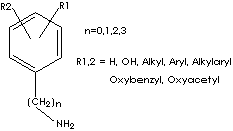
|