| CAS
NO. |
59-05-2
(Base)
15475-56-6 (Hydrochloride)
7413-34-5 (Dihydrochloride salt)
34378-65-9 (Dimethyl Ester) |
|
| EINECS NO. |
200-413-8 |
| FORMULA |
C20H22N8O5 |
| MOL
WT. |
454.44 |
|
H.S.
CODE
|
|
|
TOXICITY
|
|
| SYNONYMS |
Maxtrex;
Methoblastin; Methopterin; |
|
(+)-4-Amino-10-methylfolic acid; Metatrexan; methotextrate; Methotrexate; Methyl-aminoopterin; Methylaminopterin; methylaminopterinum;
Metotrexato; Mexate; N-4-((2,4-Diamino-6-Pteridinyl) Methyl Methylamino Benzoyl)-L-Glutamic Acid;
N-(p- (((2,4 -diamino-6-pteridyl)methyl) methylamino) benzoyl)glutamic acid;
N-bismethyl pteroylglutamic acid; Neotrexate;
N-(p(((2,4-Diamino-6-pteridinyl)methyl)- methylamino)- benzoyl)-L-glutamic acid; 4-Amino-10-methylfolic acid; 4-Amino-N10-methyl-
pteroylglutamic acid; 4-amino-N-methylpteroylglutamic acid; |
|
SMILES |
|
|
CLASSIFICATION
|
|
|
PHYSICAL AND CHEMICAL PROPERTIES
|
| PHYSICAL
STATE |
yellow
crystalline
powder |
| MELTING POINT |
|
| BOILING
POINT |
|
| SPECIFIC GRAVITY |
|
| SOLUBILITY
IN WATER |
|
| pH |
|
| VAPOR DENSITY |
|
|
AUTOIGNITION
|
|
|
NFPA
RATINGS
|
|
|
REFRACTIVE
INDEX
|
|
| FLASH
POINT |
|
| STABILITY |
Stable
under ordinary conditions. |
|
APPLICATIONS
|
Methotrexate is a folic acid antagonist blocks an enzyme needed by the cell to
live, known as antimetabolites. It inhibits the synthesis of DNA, RNA,
thymidylate, and protein. This interferes with the growth of cancer cells which
should be destroyed. Methotrexate is used as an antineoplastic in the treatment
of a wide variety of malignancies lymphocytic, meningeal, and acute myelocytic
leukemia; gestational choriocarcinoma; chorioadenoma destruens; hydatidiform
mole; carcinoma of the breast, lung, and head and neck; non-Hodgkin's lymphoma;
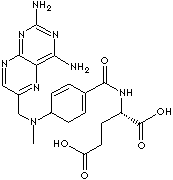
mycosis fungoides; and osteosarcoma. Chemical designation
N-[4-[[(2,4-diamino-6-pteridinyl)methyl]methylamino] benzoyl]-L-glutamic acid.
The sodium salt of methotrexate is administered orally, intrathecally,
intramuscularly, intravenously, and intra-arterially. Methotrexate can be classified
in:
- Nonsteroidal abortifacient agent
- Abortifacient agent
- Antimetabolite
- Antineoplastic
- Antirheumatic
- Dermatologic agent
- Dihydrofolate reductase inhibitor
- Folic acid antagonist
- Immunosuppressive
- Nucleic acid synthesis inhibitor
|
| SALES
SPECIFICATION (USP24) |
|
APPEARANCE
|
yellow crystalline
powder |
|
IDENTIFICATION
|
Complies
|
|
ASSAY
(HPLC) |
98.0%
- 102.0% |
|
OPTICAL ROTATION |
+19°
~ +24°
|
|
RESIDUE
ON IGNITION
|
0.1%
max
|
|
WATER
|
12.0%
max
|
|
HEAVY METALS |
10ppm
max
|
|
INDIVIDUAL
IMPURITY
|
0.5%
max (total impurity: 2.0% max)
|
|
SOLUTION CLARITY |
clear, colorless |
| TRANSPORTATION |
| PACKING |
|
| HAZARD CLASS |
|
| UN
NO. |
|
| OTHER
INFORMATION |
|
Hazard Symbols: , Risk Phrases: , Safety Phrases:
22-24/25 |
