| CAS
NO. |
57149-07-2 |
|
| EINECS
NO. |
|
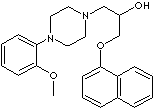
| FORMULA |
C24H28N2O3·2HCl |
| MOL
WT. |
465.42 |
|
H.S.
CODE
|
|
|
TOXICITY
|
|
| SYNONYMS |
|
| 4-(2-Methoxyphenyl)-alpha-((1-naphthalenyloxy)methyl)-1-pioerazineethanol
dichloride; (+-)-1-(4-(2-Methoxyphenyl)piperazinyl)-3-(1-naphthyloxy)propan-2-ol
dichloride; |
|
SMILES |
|
|
CLASSIFICATION
|
|
|
PHYSICAL
AND CHEMICAL PROPERTIES
|
| PHYSICAL
STATE |
white
to off-white crystalline powder
|
| MELTING
POINT |
125 - 126 C |
| BOILING
POINT |
|
| SPECIFIC
GRAVITY |
|
| SOLUBILITY
IN WATER |
Insoluble |
| pH |
|
| VAPOR
DENSITY |
|
| AUTOIGNITION |
|
| NFPA
RATINGS |
|
|
REFRACTIVE
INDEX
|
|
| FLASH
POINT |
|
| STABILITY |
Stable
under ordinary conditions. |
|
GENERAL
DESCRIPTION & APPLICATIONS
|
| Naftopidil, an alpha1-adrenoceptor antagonist, is used to treat benign prostatic
hypertrophy (BPH) which causes urinary problems by prostate gland enlargement.
It acts to smooth muscles of the prostate and thus increase urinary flow rate;
known as more effective than finasteride. Chemically it is described as
(+-)-1-(4-(2-Methoxyphenyl) piperazinyl)- 3-(1-naphthyloxy)propan-2-ol. The
dichloride salt of naftopidil is a white crystalline powder; insoluble in water,
soluble in methanol; 125 - 126 C; administered orally. |
| SALES
SPECIFICATION |
|
APPEARANCE
|
white crystalline powder |
|
IDENTIFICATION
|
Complies
Test A,B
|
| PURITY |
99.0
- 101.0%
|
|
MELTING
POINT |
125
- 126 C
|
|
HEAVY
METALS
|
10ppm
max
|
|
LOSS
ON DRYING
|
0.5%
max
|
|
RESIDUE
OF IGNITION
|
0.1%
max |
| TRANSPORTATION |
| PACKING |
|
| HAZARD
CLASS |
|
| UN
NO. |
|
| OTHER
INFORMATION |
| |
