| CAS
NO |
68373-14-8 |
|
| EINECS
NO. |
269-878-2 |
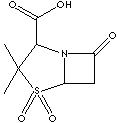
| FORMULA |
C8H11NO5S |
| MOL
WT. |
233.24 |
|
H.S.
CODE
|
|
|
TOXICITY
|
|
| SYNONYMS |
|
| (2S, 5R)-3,3-dimethyl-7-oxo-4-thia-1-azabicyclo[3.2.0]
heptane-2-carboxylate
4,4-dioxide; (2S,
5R)-(3,3-dimethyl-4,4,7-trioxo-4-thia-1- azabicyclo[3.2.0] hept-2ylcarboxylate;
|
| SMILES |
|
|
CLASSIFICATION
|
|
|
PHYSICAL
AND CHEMICAL PROPERTIES
|
| PHYSICAL
STATE |
white to off-white crystalline
powder |
| MELTING
POINT |
|
| BOILING
POINT |
|
| SPECIFIC
GRAVITY |
|
| SOLUBILITY
IN WATER |
|
| pH |
|
| VAPOR
DENSITY |
|
|
REFRACTIVE
INDEX
|
|
|
NFPA
RATINGS
|
|
|
AUTOIGNITION
|
|
| FLASH
POINT |
|
| STABILITY |
Stable
under ordinary conditions. |
|
APPLICATIONS
|
| Sulbactam, a derivative of the basic penicillin nucleus, is a beta-lactamase
inhibitor. Chemical designation is (2S,
5R)-3,3-dimethyl-7-oxo-4-thia-1-azabicyclo[3.2.0] heptane-2-carboxylate
4,4-dioxide. It is used to increase the antibacterial spectrum of penicillins
and cephalosporins against penicillinase-producing and beta-lactamase–producing
organisms such as Staphylococcus aureus, H. flu, Moraxella catarrhalis that are
resistant to ampicillin alone. Sulbactam does not improve enterococcal activity of ampicillin.
Aampicillin/Gentamicin combination is used for the treatment
of enterococcal infection. Sultamicillin is the combination of ampicillin
and sulbactam. Sultamicillin is a white to off-white crystalline powder; soluble in aqueous
diluents to yield reconstitution of ampicillin and sulbactam. Beta-lactamase enzymes are produced by some bacteria and have resistance ability
to beta-lactam antibiotics. These enzymes deactivate the antibacterial
properties of beta-lactam antibiotics by opening the beta-lactam ring.
Beta-lactamase inhibitors have little antimicrobial functions themselves and are
combined with an actual beta-lactam antibiotic. Beta-lactamase inhibitors'
function is to bind beta-lactamase enzymes to form an inactive molecule so that
the actual beta-lactam antibiotic access to the bacterial wall which it
destroys. Beta-lactamase inhibitors include clavulanic acid, sulbactam, and
tazobactam. |
| SALES
SPECIFICATION |
|
APPEARANCE
|
white to off-white crystalline
powder |
|
ASSAY
|
97.0
- 103.0%
|
|
OPTICAL
ROTATION
|
+242°
~ +253°
|
|
WATER
|
1.0%
max
|
| TRANSPORTATION |
| PACKING |
5kgs
in drum
|
| HAZARD
CLASS |
|
| UN
NO. |
|
| OTHER
INFORMATION |
|
|
