PRODUCT IDENTIFICATION
H.S. CODE
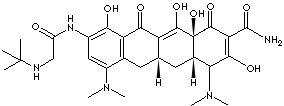
SMILES
CC(C)(C)NCC(=O)Nc1cc(c2c(c1O)C(=O)C3=C([C@]4([C@@H] (C[C@ @H] 3C2)[C@@H](C(=C(C4=O)C(=O)N)O)N(C) C)O)O)N(C)C
CLASSIFICATION
EXTRA NOTES
PHYSICAL AND CHEMICAL PROPERTIES
AUTOIGNITION
NFPA RATINGS
REFRACTIVE INDEX
Stable under ordinary conditions.
GENERAL DESCRIPTION AND APPLICATIONS
Wikipedia Linking: http://en.wikipedia.org/wiki/Tigecycline
Tigecycline is an antibiotic that is used intravenously for the treatment of infections of the skin and abdominal organs caused by bacteria that are susceptible to it. Tigecycline is similar to tetracycline antibiotics and has activity against a large number of bacteria. Tigecycline binds to bacterial ribosomes, organelles inside the bacteria that produce the cell's proteins. The binding prevents the bacterial ribosomes from producing important proteins that are needed for growth and multiplication of the bacteria. Tigecycline prevents a bacterium from multiplying, but it does not kill the bacterium. Tigecycline was approved by the FDA in June, 2005. (http://www.medicinenet.com/)Tigecycline, a derivative of the tetracycline (Some Trade Names ACHROMYCIN V, TETRACYN, TETREX) minocycline (Some Trade Names, is the first available glycylcycline. Tigecycline inhibits protein synthesis by binding to the 30S ribosomal subunit. It is bacteriostatic. Tigecycline is given IV. Tigecycline has a large volume of distribution (> 12 L/kg), penetrating well into bone, lung, liver, and kidney tissues. A half-life of 36 h should provide for once/day dosing. Most of the drug is excreted in bile and feces. Tigecycline is effective against many resistant bacteria, including those with resistance to tetracyclines. Tigecycline is active against
- Many gram-positive bacteria, including methicillin-susceptible and methicillin-resistant S. aureus, Streptococcus pneumoniae with reduced penicillin-sensitivity, vancomycin (Some Trade Names-sensitive Enterococcus faecalis, -resistant E. faecium,and Listeria sp
- Many gram-negative bacteria, such as multidrug-resistant Acinetobacter baumannii, Stenotrophomonas maltophilia, Haemophilus influenzae, and most Enterobacteriaceae (including some strains that produce extended-spectrum ��-lactamases [ESBLs] and other strains that were carbapenem-resistant based on production of a carbapenemase or metallo-��-lactamase)
- Many atypical respiratory pathogens (chlamydiae, Mycoplasma sp), Mycobacterium abscessus, M. fortuitum, and anaerobes, including Bacteroides fragilis, Clostridium perfringens, and C. difficile (http://www.merck.com/)
Tigecycline is the first drug in the glycylcycline class of antibiotics. Although it is structurally related to minocycline, alterations to the molecule resulted in its expanded spectrum of activity and decreased susceptibility to the development of resistance when compared with other tetracycline antibiotics. Tigecycline has a broad spectrum of activity, including activity against drug-resistant gram-positive organisms. (http://www.baylorhealth.edu/)
Tetracycline antibiotics include;
- Chlortetracycline (CAS # : 57-62-5)
- Demeclocycline Hydrochloride (CAS # : 64-73-3)
- Demethylchlortetracycline (CAS # : 127-33-3)
- Dihydrostreptomycin Sesquisulfate (CAS # : 5490-27-7)
- Doxycycline (CAS # : 564-25-0)
- Duramycin (CAS # : 1391-36-2)
- Meclocycline Sulfosalicylate (CAS # : 73816-42-9)
- Methacycline Hydrochloride (CAS # : 3963-95-9)
- Minocycline (CAS # : 10118-90-8)
- Neomycin (CAS # : 1404-04-2)
- Oxytetracycline (CAS # : 6153-64-6)
- Streptomycin (CAS # : 57-92-1)
- Tetracycline (CAS # : 60-54-8)
- Vancomycin (CAS # : 123409-00-7)
APPEARANCE
CONTENT
99.0% min
WATER
3.0% max
RELATED SUBSTANCES
Isomer
Impurity: 1.5% max
Individual Impurity: 1.5% max
Total Impurity:
3.0% max