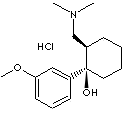
PRODUCT IDENTIFICATION
H.S. CODE
TOXICITY
CLASSIFICATION
Analgesic
PHYSICAL AND CHEMICAL PROPERTIES
Freely soluble (Freely soluble in methanol, in ethanol; slightly soluble in acetone)
REFRACTIVE INDEX
stable under ordinary conditions.
GENERAL DESCRIPTION & APPLICATIONS
Tramadol is a centrally-acting synthetic analgesic of the aminocyclohexanol group with opioid-like effects. It is not derived from natural sources, nor is it chemically related to opiates. Although pre-clinical testing has not completely explained the mode of action, at least two complementary mechanisms appear applicable: binding to µ-opioid receptors and inhibition of re-uptake of noradrenaline and serotonin. The opioid-like activity of tramadol derives from low affinity binding of the parent compound to µ-opioid receptors and higher affinity binding of the principal active metabolite, O-desmethyltramadol, denoted M1, to µ-opioid receptors. In animal models, M1 is up to 6 times more potent than tramadol in producing analgesia and 200 times more potent in µ-opioid binding. The contribution to human analgesia of tramadol relative to M1 is unknown. Both animal and human studies have shown that antinociception induced by tramadol is only partially antagonised by the opiate antagonist naloxone. In addition, tramadol has been shown to inhibit re-uptake of noradrenaline and serotonin in vitro, as have some other opioid analgesics. These latter mechanisms may contribute independently to the overall analgesic profile of tramadol. The analgesic effect is dose-dependant, but the relationship between serum concentrations and analgesic effect varies considerably between individuals. In one study, the median serum concentration of tramadol required for effective post-operative analgesia was 300 ng/mL, with individual values ranging from 20 to 990 ng/mL. Apart from analgesia, tramadol may produce other symptoms similar to that of opioids including: dizziness, somnolence, nausea, constipation, sweating and pruritus. However, tramadol causes significantly less respiratory depression than morphine. In contrast to morphine, tramadol has not been shown to cause histamine release. At therapeutic doses, tramadol has no clinically significant effect on heart rate, left ventricular function or cardiac index. Orthostatic changes in blood pressure have been observed.(http://www.medsafe.govt.nz/)
Tramadol hydrochloride (CAS 36282-47-0) is a centrally acting analgesic agent binding to mu opiate receptors. The bioavailability of a new tramadol hydrochloride injection (Limadol) was compared with a commercially available reference product by intramuscular administration in twelve healthy Chinese male volunteers by a standard two-way cross-over trial. Each volunteer received a single 100 mg injection of tramadol HCl in each phase. The bioavailability was compared using the area under the plasma concentration-time curve from time 0 to 30 h (AUC0-30), the area under the plasma concentration-time curve from time 0 to infinity (AUC0-infinity), peak plasma concentration (Cmax), and time to reach peak plasma concentration (Tmax). No statistically significant difference was observed between the Tmax, Cmax, AUC0-30 and AUC0-infinity of the two preparations. It is concluded that test and reference formulations of tramadol hydrochloride are bioequivalent for both the extent and rate of absorption after a single intramuscular injection. (http://www.ncbi.nlm.nih.gov/)
|
|
APPEARANCE
ASSAY
99.0 - 101.0%
pass IR/TLC/Test for chloride
ASH
0.1% max
HEAVY METALS
20ppm max
Individual Impurity: 0.2% max, Total Impurity: 0.4% max
0.5% max
PRICE INFORMATION