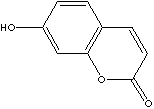
PRODUCT IDENTIFICATION
H.S. CODE
TOXICITY
CLASSIFICATION
EXTRA NOTES
PHYSICAL AND CHEMICAL PROPERTIES
225 - 234 C (Decomposes)
REFRACTIVE INDEX
EXTERNAL LINKS & GENERAL DESCRIPTION
Drug Information Portal (U.S. National Library of Medicine) - 7-Hydroxycoumarin
PubChem Compound Summary - 7-Hydroxycoumarin
KEGG (Kyoto Encyclopedia of Genes and Genomes) - Umbelliferone
http://www.ebi.ac.uk/chebi/ - Umbelliferone
http://www.ncbi.nlm.nih.gov/ - Umbelliferone
http://www.epress.com/
Plant derived phenolic coumarins might play a role as dietary antioxidants
because of their consumption in the human diet in fruits and vegetables. Umbelliferone (7-hydroxycoumarin), a derivative of coumarin,
is a benzopyrone in nature and it is present in the fruits of Aegle marmelos
Correa and Anethum graveolens L. UMB has also been reported to have antioxidant properties. The parent compound coumarin has been reported to reduce
blood glucose level. In our studies, UMB has reduced
blood glucose and lipid peroxidation and elevated antioxidants?status in plasma
and liver of STZ-diabetic rats. But, no detailed
study has been carried out on the effect of UMB on lipid peroxidation,
antioxidants and lipid profile in the heart and brain of STZ-diabetic rats.
http://www.biochemj.org/
Identification
of the human liver cytochrome P-450 responsible for coumarin 7-hydroxylase
activity
Local:
Coumarin (anhydride of o-coumaric acid) is white, crystalline lactone,
obtainable naturally from several plants, such as tonka bean, lavender, sweet
clover grass, strawberries, and cinnamon, or produced synthetically from an
amino acid, phenylalanine. It is the principle of dicumarol which inhibits the
hepatic synthesis of the vitamin K–dependent coagulation factors. Coumarin
derivatives are used widely as anticoagulants (such as warfarin, -OH group is
attached at 4 position) for the treatment of disorders in which there is excessive or
undesirable clotting, such as thrombophlebitis, pulmonary embolism, and certain
cardiac conditions. Coumarin derivatives are also used as rodenticides due
to the property of causing fatal hemorrhaging. Coumarin
has the characteristic odour like that of vanilla beans. It is used for the
preparation of perfumes, soaps, flavourings. The coumarin nucleus (benzo-2-pyrone) is derived cinnamic acid (phenylacrylic
skeleton) in the biosynthesis. Accordingly, the hydroxy group attached to
coumarin structure at 7 position is important in biosynthesis pathway.
Umbelliferone (7-hydroxy coumarin), esculetin (6,7-Dihydroxycoumarin),
scopoletin (7-hydroxy-6-methoxycoumarin) are the widespread coumarins in nature.
Umbelliferone and its derivatives are suitable for use
photoactive agents, sunscreen composition,
fluorescent
probe and dyes and used in the synthesis of drugs especially anticancer.
|
|
APPEARANCE
98.0% min
225 - 234 C
SULFATED ASH
0.2% max
LOSS ON DRYING
0.5% max
HAZARD OVERVIEW
OSHA Hazards:Irritant
GHS
PICTOGRAMS

HAZARD STATEMENTS
H315 Causes skin irritation.
H319 Causes serious eye irritation.
H335
May cause respiratory irritation.
PRECAUTIONARY STATEMENTS
P261 Avoid breathing dust/fume/gas/mist/vapours/spray.
P264
Wash skin thoroughly after handling.
P271 Use only outdoors or
in a well-ventilated area.
P280 Wear protective gloves/eye protection/face
protection.
P302 + P352 IF ON SKIN: Wash with plenty of soap
and water.
P304 + P340 IF INHALED: Remove victim to fresh air
and keep at rest in a position comfortable for breathing.
P305
+ P351 + P338 IF IN EYES: Rinse cautiously with water for several
minutes. Remove contact lenses, if present and easy to do. Continue
rinsing.
P312 Call a POISON CENTER or doctor/physician if you
feel unwell.
P321 Specific treatment (see supplemental first
aid instructions on this label).
P332 + P313 If skin irritation
occurs: Get medical advice/attention.
P337 + P313 If eye irritation
persists: Get medical advice/attention.
P362 Take off contaminated
clothing and wash before reuse.
P403 + P233 Store in a well-ventilated
place. Keep container tightly closed.
P405 Store locked up.
P501 Dispose of contents/container through a waste management company authorized
by the local government.
![]() Xi
Irritant
Xi
Irritant
RISK PHRASES
SAFETY PHRASES
26 In case of contact with eyes, rinse immediately with
plenty of water and seek medical advice
36 Wear suitable protective clothing