|
CIMETIDINE |
| Synonyms. Cimetidine; 1-Cyano-2-methyl-3-(2-(((5-methyl-4-imidazolyl)methyl)thio)ethyl)guanidine; 2-Cyano-1-methyl-3-(2-(((5-methylimidazol-4-yl)methyl)thio)ethyl)guanidine; Acibilin; Acinil; Cimal; Cimetag; Cimetidina; Cimetidinum; Cimetum; Edalene; Equaline acid reducer; Eureceptor; Evicer; Gastrobitan; Gastromet; N-Cyano-N'-methyl-N''-(2-(((5-methyl-1H-imidazol-4-yl)methyl)thio) ethyl)guanidine; Tagamet; Tametin; Tratul; Ulcedin; Ulcedine; Ulcimet; Ulcomet; Valmagen; |
|
|
| PRODUCT IDENTIFICATION | |
|
CAS RN |
51481-61-9, 70059-30-2 (HCl) |
|
EINECS RN |
257-232-2 |
|
FORMULA |
C10H16N6S |
|
MOLE WEIGHT |
252.34 |
|
H.S CODE |
2933.29.4500 |
|
SMILES |
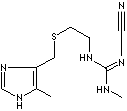
c1(c([nH]cn1)C)CSCCN\C(=N\C#N)NC |
|
CLASSIFICATION |
H2 eceptor antagonist, Anti-ulcer agent. |
|
EXTRA NOTES |
A histamine congener, it competitively inhibits histamine binding to histamine H2 receptors. Cmetidine has a range of pharmacological actions. it inhibits gastric acid secretion, as well as pepsin and gastrins output. it also blocks the activity of cytochrome P-450 which might explain proposals for use in neoadjuvant therapy. H2 histamine receptor antagonist; I1 imidazoline receptor agonist; anti-ulcer agent. Blocks cancer metastasis by inhibiting the expression of E-selectin on the surface of endothelial cells, thus blocking tumor cell adhesion. |
|
|
| PHYSICAL AND CHEMICAL PROPERTIES |
|
|
PHYSICAL STATE. |
white powder |
MELTING POINT |
142 C |
|
BOILING POINT |
|
|
DENSITY |
|
|
SOLUBILITY IN WATER |
9380 mg/l |
| SOLVENT SOLUBILITY | |
|
VAPOR DENSITY |
|
|
log P(octanol-water) |
0.417 |
|
VAPOR PRESSURE |
5.50E-09 |
|
AUTOIGNITION TEMP |
|
| pKa |
6.8 |
|
REFRACTIVE INDEX |
|
|
FLASH POINT |
|
|
| STABILITY AND REACTIVITY | |
| STABILITY | Stable under normal conditions. |
|
INCOMPATIBLE MATERIALS |
Strong oxidizing agents |
| POLYMERIZATION |
Has not been reported |
|
NFPA RATINGS |
Health: 2, Flammability:0, Reactivity: 0 |
|
|
| EXTERNAL LINKS & GENERAL DESCRIPTION |
|
Wikipedia Linking - Cimetidine Google Scholar Search - Cimetidine Drug Information Portal (U.S. National Library of Medicine) - Cimetidine PubChem Compound Summary - Cimetidine Drug Bank - Cimetidine KEGG (Kyoto Encyclopedia of Genes and Genomes) - Cimetidine http://www.ebi.ac.uk/ - Cimetidine http://www.ncbi.nlm.nih.gov/ - Cimetidine http://www.sigmaaldrich.com/Cimentidine is a competitive histamine H2-receptor antagonist whose effects include inhibition of gastric acid secretion and reduction of pepsin output. It also binds to cytochrome P450, which can lead to the inhibition of breakdown of compounds metabolized by the cytochrome P450 system. A review of the effects of cimetidine and other compounds on transport proteins has been published. Cimeditine has been used in the culture of rabbit gastric parietal cells for subsequent ultrastructural examination. Cimetidine blocks the histamineinduced surface expression of P-selectin in primary cultured human brain microvessel endothelial cells. A study of cultured human monocytes has utilized cimetidine to probe the expression of histidine decarboxylase. The susceptibility of cimetidine to metabolism by colonic bacteria in vitro has been investigated. http://dermatology-s10.cdlib.org/ |
|
|
| SALES SPECIFICATION | |
|
APPEARANCE |
white powder |
|
ASSAY |
98.0% ~ 102.0% |
|
MELTING POINT |
140 ~ 145 C |
|
LOSS ON DRYING |
0.5% max |
|
ASH |
0.1% max |
|
HEAVY METALS |
20ppm max |
|
|
| TRANSPORT & REGULATORY INFORMATION |
|
|
UN NO. |
Not regulated |
| HAZARD CLASS |
|
| PACKING GROUP | |
|
|
| SAFETY INFORMATION |
|
|
HAZARD OVERVIEW |
Target Organ Effect, Reproductive hazard. Target Organs:Kidney, Heart, Nerves., Male reproductive system. |
|
GHS |
|
|
SIGNAL WORD |
Danger |
|
PICTOGRAMS |
|
|
HAZARD STATEMENTS |
H360 |
|
P STATEMENTS |
P201-P308 + P313 |
| EC DIRECTIVES |
|
| HAZARD CODES |
|
|
RISK PHRASES |
60 |
|
SAFETY PHRASES |
26-36/37/39-45 |
|
|
| PACKING |
|
|
|
|
| PRICE INFORMATION |
|
|