COLCHICINE
PRODUCT IDENTIFICATION
H.S. CODE
TOXICITY
Oral, mouse: LD50 = 5886 ug/kg
7-alpha-H-Colchicine; 7alphaH-Colchicine; Colchisol; Colchysat;
(S)-N-(5,6,7,9-Tetrahydro-1,2,3,10-tetramethoxy-9-oxobenzo(a)heptalen-7-yl)acetamide; 1,2,3,10-Tetramethoxy-7-acetamido-6,7-dihydrobenzo(a)heptalen-9(5H)-one; Colchicin; Colchicina; Colchicine; Colchicinum; Colchineos; Colcin; Colcrys; Colsaloid; Condylon; Goutnil; Kolkicin; N-(5,6,7,9-Tetrahydro-1,2,3,10-tetramethoxy-9-oxobenzo(a)heptalen-7-yl)acetamide; N-Acetyl trimethylcolchicinic acid methylether; Other RN: 5843-86-7, 30512-31-3
SMILES
CLASSIFICATION
Antimitotic, Antirheumatic, Gout suppressant, Mitosis Modulator, Tubulin Modulator
PHYSICAL AND CHEMICAL PROPERTIES
45 g/l at 20 C
SOLVENT SOLUBILITY
REFRACTIVE INDEX
NFPA RATINGS
Health: 3, Flammability: 1, Reactivity: 0
AUTOIGNITION
FLASH POINT
EXTERNAL LINKS & GENERAL DESCRIPTION
Drug Information Portal (U.S. National Library of Medicine) - Colchicine
PubChem Compound Summary - Colchicine
IPCS INCHEM - Colchicine
Drug Bank - Colchicine
KEGG (Kyoto Encyclopedia of Genes and Genomes) - Colchicine
http://www.ebi.ac.uk/chebi/ - Colchicine
http://www.ncbi.nlm.nih.gov/ - Colchicine
The Emergency Response Safety and Health Database - Colchicine
Local:
Colchicine is a tricyclic
alkaloid prepared from Colchicum autumnale L. and found also in other Colchicum
species. Colchicine inhibits microtubule polymerization by binding to tubulin.
It is a drug used to prevent painful attacks of joint
inflammation due to deposits of uric acid in the joint (gouty
arthritis).and febrile attacks in familial Mediterranean fever. The mechanism
is not clear, but it is considered that colchicine helps to reduce
lactic acid production by leukocytes and reduce phagocytosis, with abatement of the inflammatory response.
Colchicine has other pharmacologic effect in secondary amyloidosis
(AA), and
scleroderma. Colchicine is a pale yellow powder soluble in water. The chemical
designation is N-[(7S)-1,2,3,10-tetramethoxy-9-oxo-6,7-dihydro-5H-benzo[d]heptalen-7-yl]acetamide.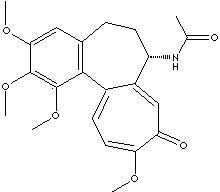
Antigout drugs include:
|
Product |
CAS RN. |
|
Probenecid |
|
|
Sulfinpyrazone |
|
| Indomethacin | 53-86-1 |
| Colchicine | 64-86-8 |
| Allopurinol | 315-30-0 |
| Naproxen | 22204-53-1 |
| Pranoprofen | 52549-17-4 |
| Amflutizole | 82114-19-0 |
| Febuxostat | 144060-53-7 |
APPEARANCE
CONTENT
97.0 - 101.0%
LOSS ON DRYING
1.0% max
1544
HAZARD OVERVIEW
GHS
PICTOGRAMS


HAZARD STATEMENTS
H300
Fatal if swallowed
H340 May cause genetic defects
PRECAUTIONARY STATEMENTS
P201
Obtain special instructions before use
P301 + P310 IF
SWALLOWED: Immediately call a POISON CENTER or doctor/physician
P308
+ P313 IF exposed or concerned: Get medical advice/attention
![]() T+
Very Toxic
T+
Very Toxic
RISK PHRASES
28
Very toxic if swallowed.
46 May cause heritable genetic
damage.
SAFETY PHRASES
13 Keep away from food, drink and animal feeding stuffs
45
In case of accident or if you feel unwell, seek medical
advice immediately (show label where possible)