|
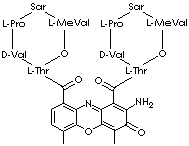
DACTINOMYCIN |
| Synonyms. Dactinomycin; Actinomycin D; 2-Amino-N,N'- bis(hexadecahydro-2,5,9-trimethyl-6,13- bis(1-methylethyl)- 1,4,7,11,14-pentaoxo-1H-pyrrolo (2,1-i) (1,4,7,10,13)oxatetra- azacyclohexadecin- 10-yl)-4,6-dimethyl-3-oxo-3H- phenoxazine-1,9-dicarboxamide; ACT D; Actinomycin 11 cosmegen; Actinomycin 7; Actinomycin A IV; Actinomycin Aiv; Actinomycin C1; Cosmegen Lyovac; Dactinomicina; Dactinomycin D; Dactinomycine; Dactinomycinum; Dilactone actinomycin D acid; Dilactone actinomycindioic D acid; Lyovac cosmegen; Lyovac-Cosmegen; Meractinomycin; |
|
|
| PRODUCT IDENTIFICATION | |
|
CAS RN |
50-76-0 |
|
EINECS RN |
200-063-6 |
|
FORMULA |
C62H86N12O16 |
|
MOLE WEIGHT |
1255.42 |
|
H.S CODE |
2941.90.1050 |
|
SMILES |
Cc1ccc(c2c1oc-3c(c(=O)c(c(c3n2)C (=O)N[C@H]4[C@H](OC(=O)[C@@ H](N(C (=O)CN(C(=O)[C@@H]5C CCN5 C(=O) [C@H](NC4=O)C(C)C)C) C)C(C)C)C)N)C)C(=O)N[C@H]6[C@H] (OC(=O)[C@@H](N(C(=O)CN(C(=O) [C@@H] 7CCCN7C(=O)[C@H](NC6=O) C(C)C)C)C)C(C)C)C |
|
CLASSIFICATION |
Antibacterial, Anti-Infective, Nucleic acid synthesis inhibitor, Protein synthesis inhibitor, Polypeptide antibiotic |
|
EXTRA NOTES |
A compound composed of a two cyclic peptides attached to a phenoxazine that is derived from streptomyces parvullus. It binds to DNA and inhibits RNA synthesis (transcription), with chain elongation more sensitive than initiation, termination, or release. As a result of impaired mRNA production, protein synthesis also declines after dactinomycin therapy. Overall Carcinogenic Evaluation: Group 3 |
|
|
| PHYSICAL AND CHEMICAL PROPERTIES | |
|
PHYSICAL STATE |
white crystalline powder |
|
MELTING POINT |
251 - 253 C |
|
BOILING POINT |
|
|
DENSITY |
|
|
SOLUBILITY IN WATER |
Insoluble |
| SOLVENT SOLUBILITY | Soluble in ethanol, isopropanol, slightly soluble in methanol |
|
VAPOR DENSITY |
|
|
log P(octanol-water) |
-0.91 |
|
VAPOR PRESSURE |
|
|
AUTOIGNITION TEMP |
|
| pH |
|
|
REFRACTIVE INDEX |
|
|
FLASH POINT |
|
|
|
| STABILITY AND REACTIVITY | |
| STABILITY | Stable under normal conditions. Hygroscopic and light sensitive |
|
INCOMPATIBLE MATERIALS |
Strong acids, Strong bases, Strong oxidizing agents, Exposure to moisture |
| POLYMERIZATION |
Has not been reported |
|
NFPA RATINGS |
Health: 3,Flammability: 0, Reactivity: 1 |
|
|
| EXTERNAL LINKS & GENERAL DESCRIPTION | |
|
Wikipedia Linking - Dactinomycin Google Scholar Search - Dactinomycin Drug Information Portal (U.S. National Library of Medicine) - Dactinomycin PubChem Compound Summary - Dactinomycin Drug Bank - Dactinomycin KEGG (Kyoto Encyclopedia of Genes and Genomes) - Dactinomycin http://www.ebi.ac.uk/ - Dactinomycin http://www.ncbi.nlm.nih.gov/ - Dactinomycin http://www.bccancer.bc.ca/ http://www.sigmaaldrich.com/
|
|
|
| SALES SPECIFICATION |
|
|
APPEARANCE |
white crystalline powder |
| ASSAY | 98.0% ~ 102.0% |
| LOSS ON DRYING |
2.0% max |
| RESIDUE ON IGNITION |
0.5% max |
|
RELATED SUBSTANCES |
2.0% max |
| HEAVY METALS |
20ppm max |
| ABSORPTION | 325nm ~ 327nm |
| ABSORBANCE |
0.32 ~ 0.38 (Ratio A1/A2) |
|
SOLUTION STATE |
clear blue (in Ethanol with KOH), clear yellow (in Ethanol with Sodium Hydrosulfite), |
| WATER CONTENT | 0.5% max |
|
|
| TRANSPORT & REGULATORY INFORMATION | |
|
UN NO. |
3462 |
| HAZARD CLASS |
6.1 |
| PACKING GROUP | II |
|
|
| SAFETY INFORMATION |
|
|
HAZARD OVERVIEW |
Highly Toxic (USA). Very toxic by inhalation, in contact with skin and if swallowed. Irritating to eyes, respiratory system and skin. Possible sensitizer. Target organ(s): Liver. Bone marrow. Light sensitive. Moisture sensitive. Cause eye irritation. Causes skin irritation. May be harmful if absorbed through the skin. May cause irritation of the digestive tract. May be harmful if swallowed. Causes respiratory tract irritation. May be harmful if inhaled. |
|
GHS |
|
|
SIGNAL WORD |
Danger |
|
PICTOGRAMS |
|
|
HAZARD STATEMENTS |
H300-H310-H315-H319-H330-H335 |
|
P STATEMENTS |
P260-P264-P280-P284-P301 + P310-P302 + P350 |
| EC DIRECTIVES |
|
| HAZARD CODES |
|
|
RISK PHRASES |
26/27/28-36/37/38 |
|
SAFETY PHRASES |
26-28-36/37-45 |
|
|
| PACKING |
|
|