PRODUCT IDENTIFICATION
H.S. CODE
TOXICITY
CLASSIFICATION
PHYSICAL AND CHEMICAL PROPERTIES
REFRACTIVE INDEX
NFPA RATINGS
AUTOIGNITION
FLASH POINT
GENERAL DESCRIPTION & APPLICATIONS
APPEARANCE
ASSAY
98.5 - 101.0% (dry basis)
pH
3.0 - 5.5
ASH
0.5% max
|
|
|
| EPINASTINE HYDROCHLORIDE | |||
|
PRODUCT IDENTIFICATION |
|||
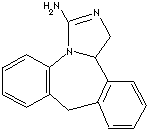
| CAS NO. | 80012-43-7 (parent), 108929-04-0 (hydrochloride) |
| |
| EINECS NO. | |||
| FORMULA | C16H15N3.HCl | ||
| MOL WT. | 285.77 | ||
|
H.S. CODE |
|||
|
TOXICITY |
|||
| SYNONYMS | Epinastina; Epinastinum; (+-)-Epinastine hydrochloride; | ||
| Epinastine monohydrochloride; 3-Amino-9,13b-dihydro-1H-dibenz(c,f)imidazo(1,5-a)azepine hydrochloride; 1H-Dibenz(c,f)imidazo(1,5-a)azepin-3-amino, 9,13b-dihydro- hydrochloride; Other CAS RN.: 134507-59-8; 80012-44-8; | |||
| DERIVATION |
|
||
|
CLASSIFICATION |
|||
|
PHYSICAL AND CHEMICAL PROPERTIES |
|||
| PHYSICAL STATE | white to yellow crystalline powder | ||
| MELTING POINT | 270 C | ||
| BOILING POINT | |||
| SPECIFIC GRAVITY | |||
| SOLUBILITY IN WATER | |||
| pH | |||
| VAPOR DENSITY |
|
||
|
REFRACTIVE INDEX |
|||
|
NFPA RATINGS |
|||
|
AUTOIGNITION |
| ||
|
FLASH POINT |
| ||
| STABILITY | Stable under normal conditions. | ||
|
GENERAL DESCRIPTION & APPLICATIONS |
|||
|
Epinastine is a histamine
H1 antagonist that blocks the actions of endogenous histamine by selectively binding to but not activating histamine H1 receptors.
Such agents also include the
classical antihistamines that antagonize or prevent the action of histamine
mainly in immediate hypersensitivity. These agents also have sedative,
anticholinergic,
and antiemetic
effects. They are used for the relief
of allergic symptoms and as antiemetics,
antivertigo agents, sedatives,
and antidyskinetics
in parkinsonism. Epinastine is used in eye drops to treat allergic conjunctivitis.
|
|||
| SALES SPECIFICATION | |||
| EPINASTINE.HCl | |||
|
APPEARANCE |
white to yellow crystalline powder | ||
| IDENTIFICATION | Complies | ||
|
ASSAY |
98.5 - 101.0% (dry basis) |
||
|
pH |
3.0 - 5.5 |
||
|
ASH |
0.5% max |
||
| TRANSPORTATION | |||
| PACKING | | ||
| HAZARD CLASS | |||
| UN NO. | |||
| OTHER INFORMATION | |||
|
|
|