PRODUCT IDENTIFICATION
57918-25-9 (parent)
56390-09-1 (hydrochloride)
H.S. CODE
TOXICITY
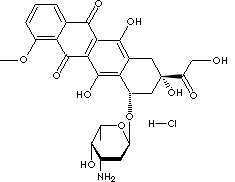
O1[C@@H](C)[C@@H]([C@H](C[C@@H]1O[C@@H]1c2c(c (c3C(c4 cccc (c4 C (c3c2O)=O)OC)=O)O)C[C@](C(CO)=O) (O)C1)N)O.Cl
CLASSIFICATION
Anthracycline, Topoisomerase II Inhibitor, Antibiotics, Antineoplastic
An anthracycline which is the 4'-epi-isomer of doxorubicin. The compound exerts its antitumor effects by interference with the synthesis and function of DNA.
PHYSICAL AND CHEMICAL PROPERTIES
REFRACTIVE INDEX
NFPA RATINGS
AUTOIGNITION
FLASH POINT
EXTERNAL LINKS & GENERAL DESCRIPTION
Wikipedia Linking - Epirubicin
Google Scholar Search - Epirubicin
Drug Information Portal (U.S. National Library of Medicine) - Epirubicin
PubChem Compound Summary - Epirubicin HCl
Drug Bank - Epirubicin
KEGG (Kyoto Encyclopedia of Genes and Genomes) - Epirubicin
http://www.ebi.ac.uk/ - Epirubicin
http://www.ncbi.nlm.nih.gov/ - Epirubicin
http://toxnet.nlm.nih.gov/
Hazardous Substances Data Bank - Epirubicin
http://www.sigmaaldrich.com/
Epirubicin is antimitotic and cytotoxic. It inhibits nucleic acid and protein synthesis. Epirubicin may do so by forming complexes with DNA and intercalation between base pairs, by inhibiting topoisomerase II activity by stabilizing the DNA-topoisomerase II complex, and by preventing the religation portion of the ligation-religation reaction that topoisomerase II catalyzes. It inhibits DNA helicase activity.
Local
Anthracycline: a class of chemotherapy drugs that are also antibiotics having a four-ring nucleus (tetrahydrotetrahydrotetracenedione.) to which is attached by aminoglycoside linkage including daunosamine (CAS:26548-47-0), isolated from the fungus Streptomyces peucetius or S. coeruleorubidus. The molecule is amphoteric. The ring-system nucleus is lipophilic, but abundant hydroxyl groups at the saturated end of the ring system is hydrophilic. The molecule contains acidic function in the ring phenolic groups as well as a basic function in the amino group in glycoside. Anthracyclines act to terminate cancer cells function by DNA intercalation, metal ion chelation, and the generation of free radicals. They are used to treat a wide range of cancers including leukemia and other neoplasms.
Epirubicin, an anthracycline cytotoxic agent, is the 4'-epi-isomer of doxorubicin, It has the same actions as doxorubicin, but has different spatial orientation of the hydroxyl group at the 4' carbon of the sugar, which features fewer side-effects and lower toxicity. The IUPAC name is (7S,9S)-7-[(2S,4S,5R,6S)-4- amino-5- hydroxy- 6-methyloxan-2-yl]oxy- 6,9,11-trihydroxy-9-(2-hydroxyacetyl)-4 -methoxy-8,10-dihydro- 7H-tetracene- 5,12-dione.
Anthracycline |
CAS RN. |
|
eta-Isorhodomycinone |
477-53-2 |
| Rhodomycin | 1401-16-7 |
| Nogalamycin | 1404-15-5 |
| Retamycin | 11130-68-0 |
| Beromycin, anthracycline | 12674-15-6 |
| Requinomycin | 12770-40-0 |
| Daunorubicin | 20830-81-3 |
| Rhodomycinone | 21288-60-8 |
| Doxorubicin | 23214-92-8 |
| Doxorubicin hydrochloride | 25316-40-9 |
| Daunosamine | 26548-47-0 |
| Pillaromycin A | 30361-37-6 |
| Carminomycin | 39472-31-6 |
| Carubicin | 50935-04-1 |
| Cosmomycin C | 55945-22-7 |
| Epirubicin | 56420-45-2 |
| Aclarubicin | 57576-44-0 |
| Idarubicin | 58957-92-9 |
| Bohemic acid complex | 64296-23-7 |
| Menogaril | 71628-96-1 |
| Pirarubicin | 72496-41-4 |
| Iremycin | 75634-51-4 |
| Cosmomycin B | 77517-27-2 |
| Sulfurmycinone | 78173-88-3 |
| Auramycinone | 78173-89-4 |
| Sulfurmycin A | 78173-90-7 |
| Auramycin B | 78173-91-8 |
| Auramycin A | 78173-92-9 |
| Sulfurmycin B | 78193-30-3 |
| Rubeomycin B | 78366-46-8 |
| 2-Hydroxyaklavinone | 79008-78-9 |
| Roseorubicins | 79392-32-8 |
| Taurimycin | 79819-02-6 |
| 2-Hydroxyaclacinomycin B | 85819-82-5 |
| Anthrapyrazole | 91440-30-1 |
| Annamycin | 92689-49-1 |
| Cosmomycin B' | 103470-57-1 |
| Cosmomycin A' | 103470-58-2 |
| Oxaunomycin | 105615-58-5 |
| Viriplanin A | 106153-36-0 |
| Moflomycin | 107430-03-5 |
| Barminomycin II | 108089-33-4 |
| Barminomycin I | 108147-17-7 |
| Nemorubicin | 108852-90-0 |
| Amrubicin hydrochloride | 110311-30-3 |
| 2-Pyrrolinodoxorubicin | 175795-76-3 |
APPEARANCE
IDENTIFICATION
positive (Test IR)
ASSAY
97.0 - 102.0%
HEAVY METALS
10ppm max
0.1% max
4.0 - 5.5
Not regulated
HAZARD OVERVIEW
May be harmful if inhaled. May cause respiratory tract irritation. Skin Harmful if absorbed through skin. May cause skin irritation. May cause eye irritation. Harmful if swallowed.
GHS
Warning
PICTOGRAMS

HAZARD STATEMENTS
H302
P STATEMENTS
P201-P308 + P313
![]() Xn
Xn
RIS PHRASES
22
SAFETY PHRASES