IFOSFAMIDE
PRODUCT IDENTIFICATION
H.S. CODE
TOXICITY
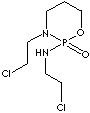
SMILES
C1[N@@]([P@@](OCC1)(NCCCl)=O)CCCl
CLASSIFICATION
Nitrogen mustard, Antineoplastic agents (alkylating)
EXTRA NOTES
Positional isomer of cyclophosphamide which is active as an alkylating agent and an immunosuppressive agent.
Other RN: 36341-88-5, 84711-20-6
PHYSICAL AND CHEMICAL PROPERTIES
SOLVENT SOLUBILITY
Soluble in chloroform
AUTOIGNITION
NFPA RATINGS
Health: 2, Flammability: 0, Reactivity: 0
REFRACTIVE INDEX
Stable under ordinary conditions.
GENERAL DESCRIPTION & APPLICATIONS
Ifosfamide, like cyclophosphamide, is an oxazophosphorine alkylating agent. Following activation in the liver, ifosfamide interferes with DNA through formation of phosphotriesters and DNA-DNA crosslinks, thereby inhibiting protein synthesis and DNA synthesis. Ifosfamide is cell cycle-specific, but cell cycle phase non-specific. Ifosfamide is an immunosuppressive agent. (BC Cancer Agency)
Cyclophosphamide. Our approach was aimed at obtaining cyclic TV-phosphorylation products of nor-nitrogen mustard as prodrugs. These chemically and pharmacologically inactive transport forms should offer opportunities for enzymatic activation to the cancerotoxic active form, e.g., by the incorporation of phosphoramide and phosphoric ester bonds into the molecule. These novel compounds were synthesized by reacting N,Nbis(2-chloroethyl)phosphoramide dichloride with alpha,omega-alkanolamines of various chain lengths. Further cyclic variants were produced with appropriate bifunctional alkanes yielding mono-, di-, or triamides of phosphoric acid. From the pharmacotherapeutic viewpoint, the diamides, especially the nitrogen mustard phosphoramide esters (or oxazaphosphorines), were most attractive since they were largely inactive chemically and biologically in vitro but highly active in vivo. Of the more than 1000 derivatives that were synthesized, some compounds were found to possess particularly favorable properties. Cyclophosphamide, the prototype of this class, was the first oxazaphosphorine cytostatic which accorded with theory; it was a chemically and pharmacologically inactive transport form, a prodrug of nor-N-mustard, which was therapeutically active in vivo. Table 1 shows the results of early comparative experiments on Yoshida ascites sarcoma of the rat receiving a single i.v. administration of cyclophosphamide. It is evident from Table 1 that the curative activity of cyclophosphamide is about as high as that of the reference substance chlormethine ./V-oxide, but it is far less toxic than this highly reactive nitrogen mustard derivative, the result being a distinctly larger therapeutic index. The first publications on the pharmacology of cyclophosphamide were soon followed by confirmation of its superior chemotherapeutic properties. The assessment of Sugiura et al. was that "Among 1,000 selected compounds and antibiotics tested against all or portions of the tumor spectrum (33 tumors) cyclophosphamide was the most effective." The product also rapidly attracted great attention in clinical oncology. The initial clinical results from Germany were soon confirmed and extended internationally. Even now, 30 years after its introduction,..... ( American Association for Cancer Research)
Cyclophosphamide is a serious drug used to treat very serious disease. One uses cyclophosphamide to kill cells that are causing harm, usually cancer cells or inflammatory cells. This potent cell-killing medication is a “nitrogen mustard” derivative, meaning it is related to the toxic chemical weapon “mustard gas” (so named because of its “mustard” or “horseradish” odor) used widely in the first World War. In chemotherapy, cyclophosphamide is classified as an “alkylating agent” which means it works by binding to DNA, and interfering with normal cell function. By disrupting cellular DNA, cyclophosphamide is able to kill the cell. Cells that divide rapidly (and thus replicate their DNA rapidly) are especially targeted by cyclophosphamide. This makes cyclophosphamide especially able to kill:
- Rapidly Dividing Cancer Cells
- Bone Marrow Cells such as developing blood cells
- Stimulated Lymphocytes (those engaged in proliferation and antibody production)
- Fetal Cells
- Hair Follicle Cells
- Intestinal Cells ( Mar Vista Animal Medical Center)
APPEARANCE
IDENTIFICATION
pass test A (IR), B (HPLC)
ASSAY
98.0 - 102.0%
pH
4.0 - 7.0
WATER
0.3% max
20ppm max
20ppm max
OTHERS
2-Chloroethylamine HCl: 0.25% max
Bacterial endotoxins: 0.125EU/mg max
Sterility: pass
HAZARD OVERVIEW
Causes skin irritation. May cause an allergic skin reaction. Causes serious eye irritation. May cause respiratory irritation. Suspected of causing cancer. Target Organ Effect, Skin sensitiser, Irritant. Target Organs:Liver injury may occur., Kidney, ears, Blood, Peripheral nervous system., Bone marrow, Testes., Female reproductive system.
GHS
Warning
PICTOGRAMS

HAZARD STATEMENTS
H315-H317-H319-H335-H351
P STATEMENTS
P261-P280-P305 + P351 + P33
![]() T
T
RISK PHRASES
25-36
SAFETY PHRASES
26-45