LAMOTRIGINE
PRODUCT IDENTIFICATION
84057-84-1
TOXICITY
H.S. CODE
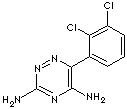
Lamotrigine; 3,5-Diamino-6-(2,3-dichlorophenyl)-as-triazine;
3,5-Diamino-6-(2,3-dichlorophenyl)-1,2,4-triazine; Lamitor; Lamotrigina; Lamotriginum; 6-(2,3-Dichlorophenyl)-1,2,4-triazine-3,5-diamine;
c1(c2c(c(ccc2)Cl)Cl)c(nc(N)nn1)N
CLASSIFICATION
Anticonvulsant, Antioxidant, Antiepileptic, Calcium channel blocker, Cardiovascular
EXTRA NOTES
Blocks voltage-gated sodium and calcium channels and inhibits release of excitatory neurotransmitters such as glutamate.
PHYSICAL AND CHEMICAL PROPERTIES
216 ~ 218 C
Insoluble
REFRACTIVE INDEX
NFPA RATINGS
Health hazard: 1, Flammability: 0, Physical hazards: 0
AUTOIGNITION
FLASH POINT
EXTERNAL LINKS & GENERAL DESCRIPTION
Drug Information Portal (U.S. National Library of Medicine) - Lamotrigine
PubChem Compound Summary - Lamotrigine
Drug Bank - Lamotrigine
KEGG (Kyoto Encyclopedia of Genes and Genomes) - Lamotrigine
http://www.ebi.ac.uk/ - Lamotrigine
http://www.ncbi.nlm.nih.gov/ - Lamotrigine
http://drugsinfo.org/LAMICTAL (lamotrigine), an antiepileptic drug (AED) of the phenyltriazine class, is chemically unrelated to existing antiepileptic drugs.Adjunctive Use: LAMICTAL is indicated as adjunctive therapy for partial seizures in adults and pediatric patients (>/=2 years of age).LAMICTAL is also indicated as adjunctive therapy for the generalized seizures of Lennox-Gastaut syndrome in adult and pediatric patients (>/=2 years of age).Monotherapy Use: LAMICTAL is indicated for conversion to monotherapy in adults with partial seizures who are receiving treatment with carbamazepine, phenytoin, phenobarbital, primidone, or valproate as the single AED.Safety and effectiveness of LAMICTAL have not been established (1) as initial monotherapy, (2) for conversion to monotherapy from AEDs other than carbamazepine, phenytoin, phenobarbital, primidone, or valproate, or (3) for simultaneous conversion to monotherapy from 2 or more concomitant AEDs (see DOSAGE AND ADMINISTRATION).Safety and effectiveness in patients below the age of 16 other than those with partial seizures and the generalized seizures of Lennox-Gastaut syndrome have not been established (see BOXED WARNING). Bipolar Disorder: LAMICTAL is indicated for the maintenance treatment of Bipolar I Disorder to delay the time to occurrence of mood episodes (depression, mania, hypomania, mixed episodes) in patients treated for acute mood episodes with standard therapy. The effectiveness of LAMICTAL in the acute treatment of mood episodes has not been established.The effectiveness of LAMICTAL as maintenance treatment was established in 2 placebo-controlled trials of 18 months' duration in patients with Bipolar I Disorder as defined by DSM-IV (see CLINICAL STUDIES, Bipolar Disorder). The physician who elects to use LAMICTAL for periods extending beyond 18 months should periodically re-evaluate the long-term usefulness of the drug for the individual patient.
http://pb.rcpsych.org/
Lamotrigine, an anticonvulsant. is licensed in the UK as monotherapy for the treatment of partial seizures and primary and secondary generalised tonic-clonic seizures. It is believed to act by inhibiting the release of excitatory neurotransmitters (glutamate and aspartate) by blocking voltage-dependent sodium channels and by stabilising presynaptic neuronal membranes (McKee & Brodie, 1996). It may also have effects on calcium channels. As well as being useful in the treatment of epilepsy, lamotrigine, like other anticonvulsants, has also been reported to be useful in the treatment of refractory bipolar
disorder and rapid-cycling bipolar disorder. A few reports of improvement in quality of life measures when used in people with epilepsy prompted the use of this drug in patients with psychiatric illness. Data about its psychotropic effects are, however, largely reported as case histories and open trials and are discussed below.
N03AX:
|
Product |
CAS RN. |
|
Sultiame |
61-56-3 |
Phenacemide |
63-98-9 |
Lamotrigine |
84057-84-1 |
Felbamate |
25451-15-4 |
Topiramate |
97240-79-4 |
Gabapentin |
60142-96-3 |
Pheneturide |
90-49-3 |
Levetiracetam |
102767-28-2 |
Zonisamide |
68291-97-4 |
Pregabalin |
148553-50-8 |
Stiripentol |
49763-96-4 |
Lacosamide |
175481-36-4 |
Carisbamate |
194085-75-1 |
Retigabine |
150812-12-7 |
Perampanel |
380917-97-5 |
Beclamide |
501-68-8 |
APPEARANCE
IDENTIFICATION
passes Test IR, UV, HPLC
ASSAY
98.0% min
216 ~ 218 C
LOSS ON DRYING
0.5% max
RESIDUE ON IGNITION
0.1% max
HEAVY METALS
20ppm max
RELATED SUBSTANCE
0.2% max
2811
HAZARD OVERVIEW
Toxic by ingestion.
GHS
Danger
PICTOGRAMS

HAZARD STATEMENTS
H301
P STATEMENTS
P301 + P310
![]()
RISK PHRASES
25
SAFETY PHRASES
45