PRODUCT IDENTIFICATION
H.S. CODE
TOXICITY
LD50 Oral rat: 1,653 mg/kg
Naxidine; Nizatidina; Nizatidine;
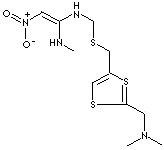
N-[2-[[2-(Dimethylaminomethyl)-1,3-thiazol-4-yl]methylsulfanyl]ethyl]-N'-methyl-2-nitroethene- 1,1-diamine; N-(2-(((2-((Dimethylamino)methyl)-4-thiazoly)methyl)thio)ethyl)-N'-methyl-2-nitro-1,1- ethenediamine; N-(2-(((2-((dimethylamino)methyl)-4-thiazolyl)methyl)thio)ethyl)-N'-methyl-2- nitro-1,1- ethenediamine; Acinon; Antizid; Axid; Calmaxid; Cronizat; Distaxid; Galitidin; Gastrax; N-(4-(6-Methylamino-7-nitro-2-thia-5-aza-6-hepten-1-yl)-2-thiazolylmethyl)-N,N-dimethylamin; Panaxid; Splendil ER; Tazac; Ulcosol; Ulxid; Zanizal; Zinga; Nizatidinum; Nizax; Nizaxid;
c1(nc(CSCCN\C(=C\[N+](=O)[O-])NC)cs1)CN(C)C
CLASSIFICATION
Anti-ulcerative, Gastrointestinal agent, Histamine H2 antagonist
EXTRA NOTES
Histamine H2 receptor antagonist. Used to treat gastroesophogeal reflux disease by preventing release of acid and pepsin into the stomach.
PHYSICAL AND CHEMICAL PROPERTIES
white to off-white crystalline powder
Soluble
REFRACTIVE INDEX
NFPA RATINGS
AUTOIGNITION
FLASH POINT
GENERAL DESCRIPTION & APPLICATIONS
Nizatidine is a histamine H2 receptor antagonist, used to treat gastric and duodenal ulcers, gastroesophogeal reflux disease by inhibiting release of acid and pepsin into the stomach. It is an off-white to buff crystalline solid with a bitter taste and mild sulfur-like odor; soluble in water. Chemical designation is N-[2-[[2-(dimethylaminomethyl)-1,3-thiazol-4-yl]methylsulfanyl]ethyl]-N'- methyl-2-nitroethene- 1,1-diamine.
|
|
APPEARANCE
white to off-white crystalline powder
ASSAY
98.5 - 100.5%
1.0% max
HEAVY METALS
10ppm max
2ppm max
RESIDUE ON IGNITION
0.1% max
LOSS ON DRYING
0.5% max
|
Total plate counmt: |
1000CFU/g max |
Moulds and yeasts: |
100CFU/g max |
Escherichia Coli: |
Negative |
Salmonella spp.: |
Negative |
TRETINOIN
5.0% max
Not regulated
HAZARD OVERVIEW
Target Organ Effect, Harmful by ingestion. Target Organs: Gastrointestinal tract
GHS
PICTOGRAMS

HAZARD STATEMENTS
H302
P STATEMENTS
![]()
RISK PHRASES
22
SAFETY PHRASES
36