|
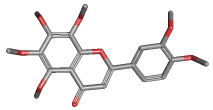
NOBILETIN |
| Synonyms. Nobiletin; 2-(3,4-Dimethoxyphenyl)-5,6,7,8-tetramethoxy-4H-1- benzopyran-4-one; 3',4',5,6,7,8-Hexamethoxyflavone; Hexamethoxyflavone; 5,6,7,8,3',4'-Hexamethoxyflavone; |
|
|
| PRODUCT IDENTIFICATION | |
|
CAS RN |
478-01-3 |
|
EINECS RN |
|
|
FORMULA |
C21H22O8 |
|
MOLE WEIGHT |
402.39 |
|
H.S CODE |
2932.99.6100 |
|
SMILES |
c12c(oc(cc2=O)c2cc(OC)c(cc2)OC)c(OC)c(c(c1OC)OC)OC |
|
CLASSIFICATION |
Flavone |
|
EXTRA NOTES |
Nobiletin is a chemical compound. It is an O-methylated flavone, a flavonoid isolated from citrus peels like in tangerine(Wikipedia) |
|
|
| PHYSICAL AND CHEMICAL PROPERTIES | |
|
PHYSICAL STATE. |
white powder |
|
MELTING POINT |
138 C |
|
BOILING POINT |
|
|
DENSITY |
|
|
SOLUBILITY IN WATER |
|
| SOLVENT SOLUBILITY | Soluble in chloroform |
|
VAPOR DENSITY |
|
|
log P(octanol-water) |
|
|
VAPOR PRESSURE |
|
|
AUTOIGNITION TEMP |
|
| pH |
|
|
REFRACTIVE INDEX |
|
|
FLASH POINT |
|
|
|
| STABILITY AND REACTIVITY | |
| STABILITY | Stable under normal conditions. |
|
INCOMPATIBLE MATERIALS |
Strong oxidizing agents, Strong bases, moisture |
| POLYMERIZATION |
Has not been reported |
|
NFPA RATINGS |
Health: 1, Flammability:0, Reactivity: 0 |
|
|
| EXTERNAL LINKS & GENERAL DESCRIPTION |
|
Wikipedia Linking - Nobiletin Google Scholar Search - Nobiletin Drug Information Portal (U.S. National Library of Medicine) - Nobiletin PubChem Compound Summary - Nobiletin KEGG (Kyoto Encyclopedia of Genes and Genomes) - Nobiletin http://www.ebi.ac.uk/ - Nobiletin http://www.ncbi.nlm.nih.gov/ - Nobiletin HMDB http://www.phytochemicals.info/ |
|
|
| SALES SPECIFICATION | |
|
APPEARANCE |
white powder |
|
CONTENT |
98.0% min |
|
LOSS ON DRYING |
5.0% max |
|
HEAVY METALS |
20ppm max |
|
MELTING POINT |
136 ~ 140 C |
|
|
| TRANSPORT & REGULATORY INFORMATION | |
|
UN NO. |
Not regulated |
| HAZARD CLASS |
|
| PACKING GROUP | |
|
|
| SAFETY INFORMATION |
|
|
HAZARD OVERVIEW |
May be harmful if inhaled. May cause respiratory tract irritation. May be harmful if absorbed through skin. May cause skin irritation. May cause eye irritation. May be harmful if swallowed. |
| HAZARD CODES |
|
|
RISK PHRASES |
|
|
SAFETY PHRASES |
26-36 |
|
|
| PACKING |
|
Preserve in light-resistant and well-closed containers |