PRODUCT IDENTIFICATION
H.S. CODE
TOXICITY
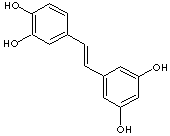
c1(c(ccc(\C=C\c2cc(O)cc(c2)O)c1)O)O
CLASSIFICATION
Stilbenoid antioxidant
EXTRA NOTES
PHYSICAL AND CHEMICAL PROPERTIES
off-white crystalline powder
226 - 230 C
AUTOIGNITION
NFPA RATINGS
REFRACTIVE INDEX
EXTERNAL LINKS & GENERAL DESCRIPTION
Wikipedia Linking - Piceatannol
Google Scholar Search - Piceatannol
Drug Information Portal (U.S. National Library of Medicine) - Piceatannol
PubChem Compound Summary - Piceatannol
Drug Bank - Piceatannol
KEGG (Kyoto Encyclopedia of Genes and Genomes) - Piceatannol
http://www.ebi.ac.uk/ - Piceatannol
http://www.ncbi.nlm.nih.gov/ - Piceatannol
http://toxnet.nlm.nih.gov/
Hazardous
Substances Data Bank - Piceatannol
Local:
Resveratrol (3,5,4'-trihydroxystilbene) is a non-flavonoid polyphenolics compound found highly in the skin of red grapes and as a constituent of red wine. It is also found in teas, berries and peanuts. Resveratrol is a compound of stilbene structure (and is responsible for the principle coloring of the plants. Resveratrol is created by plants as a phytoalexin (plant's defense arsenal) against bacteria and fungi. It is marketed as a nutritional supplement that shows health-giving antioxidant effects such as anti-cancer, antiviral, and anti-aging. Resveratrol inhibits COX-1 enzyme and demonstrates anti-inflammatory effects. It is a platelet aggregation inhibitor to block the adhesion of blood cells to vessel walls and therefore prevents heart disease and cerebral apoplexy.
It exists as two structural isomers: cis- (Z)
and trans- (E),
Piceatannol or 3,4,3',5'-tetrahydroxy-trans-stilbene is a phenolic that is structurally related to resveratrolPiceatannol has one more hydroxyl group at 3' position. It is a metabolite of resveratrol and is found in red wine. Pterostilbene (3,5-Dimethoxy-4'-hydroxy-trans-stilbene) is an analog of resveratrol. The two phenolic hydroxyl groups among three are converted to methyl ethers. Some reports say that the ethers enhance antioxidant effects.
APPEARANCE
off-white crystalline powder
98.0% min (HPLC)
LOSS ON DRYING
0.5% max
ASH
0.1% max
HEAVY METALS
20ppm max
MICROBIOLOGICAL TEST
Total Plate Count: 1000CFU/G
max
Mold and Yeast: 100CFU/G max
E.coli: Non-detective
Salmonella: Non-detective
HAZARD OVERVIEW
Not known
RISK PHRASES
SAFETY PHRASES
24/25