|
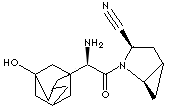
SAXAGLIPTIN |
| Synonyms. Saxagliptin; (1S,3S,5S)-2-((2S)-Amino(3-hydroxytricyclo(3.3.1.13,7)dec-1-yl)acetyl)2- azabicyclo(3.1.0)hexane-3-carbonitrile; Onglyza; |
|
|
| PRODUCT IDENTIFICATION | |
|
CAS RN |
361442-04-8 (anhydrous), 945667-22-1 (hydrate) |
|
EINECS RN |
|
|
FORMULA |
C18H25N3O2 |
|
MOLE WEIGHT |
315.41 |
|
H.S CODE |
2933.99.7500 |
|
SMILES |
N1([C@H](CC2[C@H]1C2)C#N)C(=O)[C@@H](C12CC3CC(C1)(O)CC (C3)C2)N |
|
CLASSIFICATION |
Dipeptidyl-Peptidase IV Inhibitor, Hypoglycemic agent, Protease Inhibitor |
|
EXTRA NOTES |
Putative antidiabetic agent for treating type 2 diabetes; inhibits DPP4 protein, human. Treatment of Type II diabetes mellitus and metabolic syndrome |
|
|
| PHYSICAL AND CHEMICAL PROPERTIES | |
|
PHYSICAL STATE. |
white powder |
MELTING POINT |
|
|
BOILING POINT |
548 ~ 549 C |
|
DENSITY |
1.35 |
|
SOLUBILITY IN WATER |
|
| SOLVENT SOLUBILITY | |
|
VAPOR DENSITY |
|
|
log P(octanol-water) |
|
|
VAPOR PRESSURE |
|
|
AUTOIGNITION TEMP |
|
| pKa |
|
|
REFRACTIVE INDEX |
|
|
FLASH POINT |
|
|
| STABILITY AND REACTIVITY | |
| STABILITY | Stable under normal conditions. |
|
INCOMPATIBLE MATERIALS |
Strong oxidizing agents |
| POLYMERIZATION |
Has not been reported |
|
NFPA RATINGS |
Health: 0, Flammability:0, Reactivity: 0 |
|
|
| EXTERNAL LINKS & GENERAL DESCRIPTION |
||||||||||||||||||
|
Wikipedia Linking - Saxagliptin Google Scholar Search - Saxagliptin Drug Information Portal (U.S. National Library of Medicine) - Saxagliptin PubChem Compound Summary - Saxagliptin Drug Bank - Saxagliptin KEGG (Kyoto Encyclopedia of Genes and Genomes) - Saxagliptin http://www.ebi.ac.uk/ - Saxagliptin http://www.ncbi.nlm.nih.gov/ - Saxagliptin http://toxnet.nlm.nih.gov/ http://dailymed.nlm.nih.gov/ http://www.biomedcentral.com/ Local
|
|
|
| SALES SPECIFICATION | |
|
APPEARANCE |
off-white to yellow powder |
|
ASSAY |
98.0% min |
|
LOSS ON DRYING |
5.0% max |
|
HEAVY METALS |
10ppm max |
|
|
| TRANSPORT & REGULATORY INFORMATION |
|
|
UN NO. |
Not regulated |
| HAZARD CLASS |
|
| PACKING GROUP | |
|
|
| SAFETY INFORMATION |
|
|
HAZARD OVERVIEW |
Not known |
| HAZARD CODES |
|
|
RISK PHRASES |
|
|
SAFETY PHRASES |
|
|
|
| PACKING |
|
|
|
|
| PRICE INFORMATION |
|
|