PRODUCT IDENTIFICATION
H.S. CODE
TOXICITY
Antineoplastic, Antibiotic
SMILES
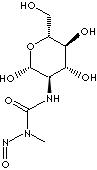
N([C@@H]1[C@H]([C@@H]([C@@H](CO)O[C@@H]1O)O)O)C(N(N=O)C)=O
EXTRA NOTES
Overall Carcinogenic Evaluation: Group 2B
PHYSICAL AND CHEMICAL PROPERTIES
SOLVENT SOLUBILITY
Soluble in lower alcohols and ketones
REFRACTIVE INDEX
NFPA RATINGS
AUTOIGNITION
FLASH POINT
EXTERNAL LINKS & GENERAL DESCRIPTION
-Properties: Streptozotocin is a mixture of α- and β-stereoisomers. It occurs as pale yellow or off-white crystals, powder, or platelets, while the research grade may be an off-white to tan solid. It is very soluble in water, ketones, and lower alcohols, slightly soluble in polar organic solvents, and insoluble in nonpolar organic solvents. The pure compound is sensitive to humidity and light. Streptozotocin decomposes to diazomethane in alkaline solutions at 0°C. When heated to decomposition, it emits toxic fumes of nitrogen oxides (IARC 1978, HSDB 2001).
-Use: Streptozotocin is primarily used in the treatment of metastasizing pancreatic islet cell tumors. It is also effective in treating malignant carcinoid tumors. It has been investigated for use in diabetes, since it has a specific toxic action on pancreatic β-cells. Streptozotocin has also been investigated as a potential antibacterial agent, but has never been used commercially for this purpose (IARC 1978).
-Production: Streptozotocin is derived from the soil microorganism Streptomyces achromogenes and has also been synthesized by three different procedures (IARC 1978). Chem Sources (2001) identified 12 current U.S. suppliers for streptozotocin. The USITC identified two domestic manufacturers of streptozotocin in 1987 and 1988, but no production volumes were reported (USITC 1988, 1989). Prior to 1983, there were two producers of streptozotocin identified by the USITC (USITC 1983). In 1979, U.S. production of streptozotocin was probably greater than 2000 lb (908 kg) (HSDB 2001). No current data on imports or exports of streptozotocin were found.
-Exposure: Health professionals such as pharmacists, doctors, and nurses may be exposed to streptozotocin while dispensing, preparing, or administering pharmaceuticals. Potential occupational exposure may occur during streptozotocin production and during the formulation of pharmaceuticals. However, this exposure is site-limited. The National Occupational Exposure Survey (1981-1983) indicated that 2,074 workers, including 1,714 women, potentially were exposed to streptozotocin. This estimate was derived from observations of the actual use of the compound (30% of total observations) and the use of trade name products known to contain the compound (70%) (NIOSH 1984).
-Regulations: EPA - Comprehensive Environmental Response, Compensation, and Liability Act Reportable Quantity (RQ) = 1 lb Resource Conservation and Recovery Act Listed Hazardous Waste: Waste codes in which listing is based wholly or partly on substance - U206 Listed as a Hazardous Constituent of Waste FDA Streptozotocin is a prescription drug subject to labeling and other requirements
(http://ntp.niehs.nih.gov/)
APPEARANCE
IDENTIFICATION
pass test A,B,C (UV Absorption, IR Spectrum, HPLC)
ASSAY
98.5% - 101.50%
0.5% max
alpha ISOMER
75.0% min
HEAVY METALS
20ppm max
RELATED SUBSTANCE
Individual Impurity: 0.5% max Total Impurity: 1.0% max
0.1% max
HAZARD OVERVIEW
May cause cancer. May be harmful if inhaled. May cause respiratory tract irritation. May be harmful if absorbed through skin. May cause skin irritation. May cause eye irritation. May be harmful if swallowed. OSHA Hazards:Carcinogen, Target Organ Effect. Target Organs:Pancreas., Liver, Kidney, Blood, Reproductive system.Pancreas., Liver, Kidney, Blood, Reproductive system
GHS
Danger
PICTOGRAMS

HAZARD STATEMENTS
H350
P STATEMENTS
P201-P308 + P313
![]() T
T
RISK PHRASES
45
SAFETY PHRASES
45-53