PRODUCT IDENTIFICATION
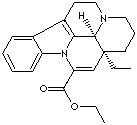
256-028-0
TOXICITY
H.S. CODE
(+)-Apovincaminic acid ethyl ester; (+)-cis-Apovincaminic acid ethyl ester; 3-alpha,16-alpha-Apovincaminic acid ethyl ester; Apovincaminate d'ethyle; Apovincaminic acid ethyl ester; Cavinton; Ethyl (+)-apovincaminate; Ethyl (+)-cis-apovincaminate; cis-Apovincaminic acid ethyl ester; (3-alpha,16-alpha)-Eburnamenine-14-carboxylic acid, ethyl ester; Other RN: 80038-06-8, 115986-87-3
CLASSIFICATION
Cardiovascular agent, Neuroprotective agent, Nootropic agent, Phosphodiesterase Inhibitor, Vasodilator agent, Cyclic Nucleotide
EXTRA NOTES
PHYSICAL AND CHEMICAL PROPERTIES
white to slightly yellow crystalline powder
Practically insoluble
Soluble in methylene chloride, slightly soluble in anhydrousethanol.
REFRACTIVE INDEX
NFPA RATINGS
Health hazard: 1, Fire: 0, Reactivity Hazard: 0
AUTOIGNITION
FLASH POINT
EXTERNAL LINKS & GENERAL DESCRIPTION
Drug Information Portal (U.S. National Library of Medicine) - Vinpocetine
http://www.ncbi.nlm.nih.gov/
Vinpocetine
is an alkaloid extracted from the periwinkle plant and has been
tested as a neuronal plasticity enhancer and marketed as a “memory
booster.” Vinpocetine treatment has been shown to facilitate long-term
potentiation, improve spatial memory in animal models, and enhance
performance on cognitive tests in humans. The cognitive enhancement
function of vinpocetine comes from its inhibition of PDE type 1,
which leads to an increase in cAMP and cGMP levels. These cyclic
nucleotides can in turn activate a series of kinases that phosphorylate
the transcription factors cAMP response element binding protein
(CREB) and serum response factor (SRF), leading to the expression
of plasticity-related genes
http://www.mskcc.org/
Mechanism
of Action: Vinpocetine has been shown to possess antioxidant and
hydroxyl radical scavenging properties in vitro. It inhibits phosphodiesterase
1 (PDE1) activity and improves cerebral blood flow by elevating
cGMP and cAMP, by increasing mitochondrial function and also by
improving glucose and oxygen utilization by the brain. Vinpocetine
helps improve spatial memory in rats through its ability to prevent
neuronal damage and to favorably modulate cholinergic function.
In an animal study, it increased cerebral microcirculation and blood
flow by inhibiting platelet aggregation. Administration of vinpocetine
to chronic stroke patients increased glucose uptake and release
in unaffected areas of the brain. Vinpocetine does not possess systemic
circulatory effects or any effects on heart rate or blood pressure.
It demonstrated antiepileptic effects by suppressing the abnormal
neuronal excitability through the regulation of sodium channels
and release of dopamine in the striatal nerve endings. Vinpocetine
can increase the effects of radiation by increasing tumor oxygenation.
It also shows anti-inflammatory effects by inhibiting TNF-alpha-induced
NF-kappaB activities
http://www.jerrycott.com/
Vinpocetine
is a derivative of Vincamine, which is an alkaloid of the common
periwinkle plant (Vinca minor). It selectively dilates the arteries
and capillaries in the head area, which improves circulation to
the brain, thus alleviating cerebral insufficiency. Ongoing research
around the world indicates that it may help improve memory, learning
ability, insomnia, hearing, eyesight, and effects of menopause,
and increase tolerance to damage caused by hypoxia (lack of oxygen,
such as occurs with a stroke or heart attack). Vinpocetine is often
used for the treatment of cerebral circulatory disorders such as
memory problems, acute stroke, aphasia (loss of the power of expression),
apraxia (inability to coordinate movements), motor disorders, dizziness,
tinnitus and other inner-ear problems, and headache. Vinpocetine
is also used to treat acute or chronic ophthalmological diseases
of various origins, with visual acuity improving in 70% of the subjects.
APPEARANCE
white to slightly yellow crystalline powder
IDENTIFICATION
passes Test A, B
ASSAY
98.5 ~ 101.5%
SPECIFIC ROTATION
+127° ~ +134°
MELTING POINT
245 ~ 255 C
IMPURITY
Individual impurity: 0.5% max
Total impurity: 1.5% max
LOSS ON DRYING
0.5% max
SULPHATED ASH
0.1% max
GHS
PICTOGRAMS

HAZARD STATEMENTS
H302 Harmful if swallowed.
PRECAUTIONARY STATEMENTS
P264 Wash skin thoroughly after handling.
P270 Do not eat,
drink or smoke when using this product.
P301 + P312 IF SWALLOWED:
Call a POISON CENTER or doctor/physician if you feel unwell.
P330
Rinse mouth.
P501 Dispose of contents/container through a waste management company authorized
by the local government.
![]() Xn
Harmful
Xn
Harmful
RISK PHRASES
22 Harmful if swallowed
SAFETY PHRASES
36 Wear suitable protective clothing