|
ZOFENOPRIL CALCIUM |
| Synonyms. Zofenopril calcium; Bifril; Zofenil; Zoprace; (4S)-1-((2S)-3-(benzoylthio)-2-methyl-1-oxopropyl)-4-(phenylthio)-L-proline calcium salt; (4S)-N-((S)-3-Mercapto-2-methylpropionyl)-4-(phenylthio)-L-proline benzoate calcium salt; |
|
|
| PRODUCT IDENTIFICATION | |
|
CAS RN |
81938-43-4 |
|
EINECS RN |
|
|
FORMULA |
C22H22NO4S2·1/2Ca |
|
MOLE WEIGHT |
448.58 |
|
H.S CODE |
2933.99.5300 |
|
SMILES |
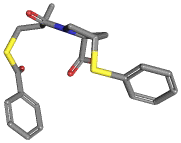
[Ca+2].C([C@H]1N(C([C@@H](CSC(=O)c2ccccc2)C)=O)C[C@H](C1)Sc1ccccc1) (=O)[O-].C([C@H]1N(C[C@H](C1)Sc1ccccc1)C([C@@H](CSC(c1ccccc1)=O)C)= O)(=O)[O-] |
|
CLASSIFICATION |
ACE inhibitor. Antihypertensive |
|
EXTRA NOTES |
Zofenopril is a long-lasting, lipophilic ACE inhibitor. Zofenopril is also an inhibitor of PEPT2, the predominant peptide transporter in kidney and choroid plexus. |
|
|
| PHYSICAL AND CHEMICAL PROPERTIES | |
|
PHYSICAL STATE. |
white crystalline powder |
|
MELTING POINT |
>250 C |
|
BOILING POINT |
|
|
DENSITY |
|
|
SOLUBILITY IN WATER |
|
| SOLVENT SOLUBILITY |
|
|
VAPOR DENSITY |
|
|
log P(octanol-water) |
3.5 |
|
VAPOR PRESSURE |
|
|
AUTOIGNITION TEMP |
|
| pH |
|
|
REFRACTIVE INDEX |
|
|
FLASH POINT |
|
|
|
| STABILITY AND REACTIVITY | |
| STABILITY | Stable under normal conditions. |
|
INCOMPATIBLE MATERIALS |
Strong acids and oxidizing agents |
| POLYMERIZATION |
Has not been reported |
|
NFPA RATINGS |
Health: 1, Flammability:0, Reactivity: 0 |
|
|
| EXTERNAL LINKS & GENERAL DESCRIPTION |
|
Wikipedia Linking - Zofenopril Google Scholar Search - Zofenopril Drug Information Portal (U.S. National Library of Medicine) - Zofenopril PubChem Compound Summary - Zofenopril KEGG (Kyoto Encyclopedia of Genes and Genomes) - Zofenopril Chemical Entities of Biological Interest (ChEBI) - Zofenopril http://www.ncbi.nlm.nih.gov/ - Zofenopril Human Metabolome Database - Zofenopril http://www.tandfonline.com/ |
|
|
| SALES SPECIFICATION | |
|
APPEARANCE |
white crystalline powder |
|
PURUTY |
98.0% min |
|
LOSS ON DRYING |
5.0% max |
|
HEAVY METALS |
20ppm max |
|
OPTICAL ROTATION |
-65° ~ -70° (c = 1 in methanol/HCl) |
|
MELTING POINT |
>250 C |
|
|
| TRANSPORT & REGULATORY INFORMATION | |
|
UN NO. |
3077 |
| HAZARD CLASS |
9 |
| PACKING GROUP | III |
|
|
| SAFETY INFORMATION | |
|
HAZARD OVERVIEW |
OSHA Hazards: No known OSHA hazards. GHS Classification: Acute aquatic toxicity (Category 1), Chronic aquatic toxicity (Category 1). Very toxic to aquatic life with long lasting effects. Inhalation: May be harmful if inhaled. May cause respiratory tract irritation. Eyes: May cause eye irritation. Skin: May be harmful if absorbed through skin. May cause skin irritation. Ingestion: May be harmful if swallowed. |
|
GHS |
|
|
SIGNAL WORD |
Warning |
|
PICTOGRAMS |
|
|
HAZARD STATEMENTS |
H410 |
|
P STATEMENTS |
P273-P501 |
| EC DIRECTIVES |
|
| HAZARD CODES |
|
|
RISK PHRASES |
50/53 |
|
SAFETY PHRASES |
60-61 |
|
|
| PACKING |
|
Preserve in light-resistant and well-closed containers |
|
|
| PRICE INFORMATION |