|
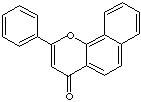
alpha-NAPHTHOFLAVONE |
| alpha-Naphthoflavone; 2-Phenyl-4H-naphtho(1,2-b)pyran-4-one; 7,8-BF; 7,8-Benzoflavone; Benzo(h)flavone; alpha-Naphthylflavone; ANF; ,2-Phenylbenzo[H]chromen-4-one; |
|
|
| PRODUCT IDENTIFICATION |
|
|
CAS RN |
604-59-1 |
|
EINECS RN |
210-071-1 |
|
FORMULA |
C19H12O2 |
|
MOLE WEIGHT |
272.30 |
|
H.S. CODE |
2932.99.6100 |
|
UN NO. |
|
|
SMILES |
c12c3c(ccc1c(cc(o2)c1ccccc1)=O)cccc3 |
|
CLASSIFICATION |
Benzopyrans, Indicator (Redox, Titration), Antioxidant, Flavonoid |
|
EXTRA NOTES |
Inhibitor of aryl hydrocarbon hydroxylase and chemical carcinogenicity. |
|
|
| PHYSICAL AND CHEMICAL PROPERTIES |
|
|
PHYSICAL STATE |
slightly yellow powder. |
|
MELTING POINT |
155 - 158 C |
|
BOILING POINT |
|
|
DENSITY |
|
|
SOLUBILITY IN WATER |
Insoluble |
|
SOLVENT SOLUBILITY |
Slightly soluble in ethanol, chloroform. Soluble in sulfuric acid |
|
pH |
|
|
VAPOR DENSITY |
|
|
REFRACTIVE INDEX |
|
|
FLASH POINT |
|
|
|
| STABILITY AND REACTIVITY | |
| STABILITY | Stable under normal conditions |
|
INCOMPATIBILITIES |
Strong oxidizing agents |
| DECOMPOSITION PRODUCTS |
Carbon oxides\ |
| POLYMERIZATION |
|
| NFPA RATING | Health: 1; Flammability: 0; Instability: 0; |
|
TOXICOLOGICAL |
|
|
|
|
POTENTIAL HEALTH EFFECTS |
|
|
HAZARD OVERVIEW |
May cause eye and skin irritation. May cause respiratory and digestive tract irritation. |
|
EYE |
May cause eye irritation. |
|
SKIN |
May be harmful if absorbed through skin. May cause skin irritation. |
|
INGESTION |
May cause irritation of the digestive tract. |
|
INHALATION |
May be harmful if inhaled. May cause respiratory tract irritation. |
| TARGET ORGANS |
No data found. |
|
|
| TRANSPORT & REGULATORY INFORMATION |
|
|
UN NO. |
|
| HAZARD CLASS |
|
| PACKING GROUP |
|
| HAZARD SYMBOL |
|
|
RISK PHRASES |
|
|
SAFETY PHRASES |
24/25 |
|
|
| EXTERNAL LINKS & GENERAL INFORMATION |
|
http://www.sciencemag.org/ http://pubs.acs.org/
|
|
|
| SALES SPECIFICATION |
|
|
APPEARANCE |
white to light yellow crystalline powder |
|
CONTENT |
95% min |
|
MELTING POINT |
155 - 158 C |
|
|
| PRICE |
| U$1,400.- (1kg) |