PRODUCT IDENTIFICATION
H.S. CODE
TOXICITY
CLASSIFICATION
Flavor, Cannabinoid, Sesquiterpene,
EXTRA NOTES
FEMA No: 2252
PHYSICAL AND CHEMICAL PROPERTIES
129 - 130 C
AUTOIGNITION
NFPA RATINGS
REFRACTIVE INDEX
96 C
Stable under ordinary conditions
GENERAL DESCRIPTION & APPLICATIONS
Drug Information Portal (U.S. National Library of Medicine) - Caryophyllene
PubChem Compound Summary - Caryophyllene
KEGG (Kyoto Encyclopedia of Genes and Genomes) - Caryophyllene
http://www.ebi.ac.uk/chebi/ - Caryophyllene
http://www.ncbi.nlm.nih.gov/ - Caryophyllene
Human Metabolome Database - Caryophyllene
http://www.ch.ic.ac.uk/
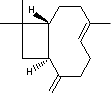
Caryophyllene, a constituent of many essential oils, include clove oil, has a
trans alkene contained in a 9-membered ring. One interesting property is that it
has 4 diastereoisomers possible, originating from a total of three disymmetric
centres present in the molecule. Two of these are conventional chiral centres,
one is present in the form of a disymmetric trans double bond. To understand why
such a bond can result in two configurations, one must appreciate that
(concurrent) rotation about the two C-C single bonds adjacent to the alkene is
in fact restricted. This phenomenon, also known as atropisomerism, is a feature
of a number of natural products, and arises from a combination of angle strain
in a medium sized ring caused by the rotation, together with trans-annular
steric effects.
Application Information: Used in spice blends and citrus flavors, especially in chewing gum. Also used in soaps and detergents.
http://ntp.niehs.nih.gov/
Metabolism: Asakawa and coworkers (1981,1986) characterized the
metabolism of beta-caryophyllene in rabbits as shown in the following
scheme.
The main metabolite, (10S)-(-)-14-hydroxycaryophyllene-5,6-oxide, was
separated as an acetate, (10S)-(-)-14-acetoxycaryophyllene-5,6-oxide, using
column chromatography by n-hexane-ethyl acetate. A second acetate, identified as
(-)-caryophyllene-5,6-oxide-2,12-diol monoacetate, was the hydroxyacetate
derivative of an additional metabolite, caryophyllene-5,6-oxide-2,12-glycol.
The metabolic pathway shown as A was confirmed by administering
(-)-caryophyllene-5,6-oxide to rabbits. After being acetylated compound IV
was identified and compound III was established as the major metabolite. The
authors note that the presence of the route B remains to be clarified and that
route A may be favorable to route B since the oxide is often found in
essential oils. The stereoselective biohydroxylation of the gem-dimethyl
group on the four-membered ring in mammals had not previously been reported.
Other Biological Effects: Several studies have documented the
anti-inflammatory, cytoprotective, and enzyme-inducing activities of
beta-caryophyllene, as well as its in vitro cytotoxicity against several
solid tumor cells.
APPEARANCE
ASSAY
90.0% min
REFRACTIVE INDEX
1.498 - 1.504
SPECIFIC GRAVITY
0.90 - 0.91
OPTICAL ROTATION
-10° ~ -5°