| CAS
NO. |
75-07-0 |
|
| EINECS
NO. |
200-836-8 |
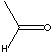
| FORMULA |
CH3CHO |
| MOL
WT. |
44.05 |
|
H.S.
CODE
|
2912.12 |
| TOXICITY |
Oral
rat LD50: 661 mg/kg |
| SYNONYMS |
Acetic
aldehyde; Acetylaldehyde; Ethylaldehyde; Ethanal; |
| Acetaldehyd;
Aldehyde acetique; Aldeide acetica; Octowy aldehyd;
Acetylaldehyde; |
| DERIVATION |
|
|
CLASSIFICATION
|
|
|
GENERAL
DESCRIPTION
|
|
Acetaldehyde,
also called Ethanal (CH3CHO), is an aldehyde used
as a starting material in the synthesis of acetic
acid, n-butyl alcohol, ethyl acetate, and other
chemical compounds. It is manufactured by the oxidation
of ethyl alcohol and by the catalytic
hydration
of acetylene (in Germany). Pure acetaldehyde is
a colourless, miscible with water,
flammable
liquid with pungent, fruity odour; it boils at 20.8
C.
|
|
PHYSICAL
AND CHEMICAL PROPERTIES
|
| PHYSICAL
STATE |
easily
volatile clear liquid |
|
MELTING
POINT |
-123
C |
| BOILING
POINT |
20
- 21 C |
| SPECIFIC
GRAVITY |
0.78
- 0.79 |
| SOLUBILITY
IN WATER |
Miscible |
| pH |
|
| VAPOR
DENSITY |
1.52 |
|
AUTOIGNITION
|
230
C
|
|
NFPA
RATINGS
|
Health:
3; Flammability: 4; Reactivity: 2 |
|
REFRACTIVE
INDEX
|
|
| FLASH
POINT |
-38
C |
| STABILITY |
Unstable
in air. May undergo autopolymerization with metals
or acids. |
|
APPLICATIONS
|
|
The
main use of acetaldehyde is as an intermediate for
the synthesis of other chemicals. The
main derivatives of Acetaldehyde are the oxygenated
solvent Ethyl Acetate, Pentaerythritol (used in
the production of synthetic resins for the paint
industry) and Pyridines. Acetaldehyde
is used in the production of perfumes, polyester
resins, and basic dyes. Acetaldehyde is also used
as a solvent in the rubber, tanning, and paper industries,
as a fruit and fish preservative, as a flavoring
agent, for hardening gelatin, as a denaturant for
alcohol and in fuel compositions.
|
| SALES
SPECIFICATION |
|
APPEARANCE
|
clear
liquid |
| PURITY |
99.7%
min
|
| COLOR,
APHA |
10
max
|
| SPECIFIC
GRAVITY |
0.78
- 0.79 (20/20 C)
|
| BOILING
POINT |
21
C
|
| PRECAUTION
IN HANDLING |
Acetaldehyde
is not compatible with strong oxidizers, acids,
alcohols, ammonia, amines, phenols, ketones, acid
anhydrides and halogens.
Acetaldehyde reacts
violently with anhydrous ammonia, hydrogen cyanide,
hydrogen sulfide and alkaline materials (such as
sodium hydroxide).
Acetaldehyde reacts with air
to form unstable peroxides which can explode.
Store
in tightly closed airtight containers in a cool,
dark, well-ventilated area.
Nitrogen or another
inert gas should be used as a "blanket"
over liquid Acetaldehyde in storage containers.
Sources
of ignition, such as smoking and open flames, are
prohibited where Acetaldehyde is handled, used,
or stored.
Metal containers involving the transfer
of Acetaldehyde should be grounded and bonded. Drums
must be equipped with self-closing valves, pressure
vacuum bungs, and flame arresters.
Use only
non-sparking tools and equipment, especially when
opening and closing containers of Acetaldehyde.
Prior to working with Acetaldehyde you should be
trained on its proper handling and storage. |
|
TRANSPORTATION
|
| PACKING |
150kgs
in drum |
| HAZARD
CLASS |
|
| UN
NO. |
|
| OTHER
INFORMATION |
| European
Hazard Symbols: XN F+, Risk Phrases: 12-36/37-40,
Safety Phrases: 16-33-36/37 |
| DESCRIPTION
OF ALDEHYDE |
Aldehydes
are organic compounds containing -CHO radical, in
which a carbon atom forms a solid bond with an
oxygen atom and is also bonded to a hydrogen atom
and another group denoted by R, which can be a second
hydrogen atom, an alkyl group, or an aryl group.
The most important and the simplest examples are
methanal (formaldehyde), HCOH, and ethanal (acetaldehyde),
CH3CHO.( In systematic chemical nomenclature,
aldehyde names end with the suffix -al). Formaldehyde
is used to make synthetic resins by reaction with
phenols, urea, and melamine, as a chemical intermediate,
as an embalming fluid, and as a disinfectant. Acetaldehyde
is used chiefly to manufacture acetic acid. They
are unpleasant-smelling liquids widely used in the
chemical industry, whereas aromatic aldehydes frequently
have pleasant smells and are used widely as flavourings
and perfumes. An example is benzaldehyde (benzenecarbaldehyde,
C6H5CHO), a derivative of
benzene with an aldehyde group attached to the ring.
It is a colourless oil smelling of almonds offer
applications to both in perfumes and flavourings.
Aldehydes are formed by oxidation of primary alcohols;
further oxidation yields carboxylic acids. Aldehydes
have certain characteristic addition and condensation
reactions. Aldehydes can be reduced to primary alcohols
(ketones to secondary alcohols). Aldehydes form
cyanohydrins with hydrogen cyanide, acetals with
alcohol, yellow-orange solid derivatives with DNP
( 2,4-dinitrophenylhydrazones) and undergo condensation
reactions to yield oximes (compounds containing
the group C:NOH), hydrazones (organic compounds
containing the group =C:NNH2), and semicarbazones
(organic compounds containing the unsaturated group
=C:N.NH.CO.NH2). Aldehydes readily polymerize.
|
