| CAS
NO. |
75-12-7 |
|
| EINECS
NO. |
200-842-0 |
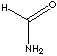
| FORMULA |
HCONH2 |
| MOL
WT. |
45.04 |
|
H.S.
CODE
|
|
| TOXICITY |
Oral rat LD50: 5577 mg/kg |
| SYNONYMS |
Methanamide;
Carbamaldehyde; Formimidic
Acid; |
| DERIVATION |
|
|
CLASSIFICATION
|
|
|
PHYSICAL
AND CHEMICAL PROPERTIES
|
| PHYSICAL
STATE |
Clear liquid with ammonia odor. |
|
MELTING
POINT |
2.5
C |
| BOILING
POINT |
210
C (Decomposes) |
| SPECIFIC
GRAVITY |
1.13
- 1.14 |
| SOLUBILITY
IN WATER |
miscible |
| pH |
7 |
| VAPOR
DENSITY |
1.6 |
|
AUTOIGNITION
|
|
|
NFPA
RATINGS
|
Health: 2; Flammability: 1; Reactivity: 0 |
|
REFRACTIVE
INDEX
|
|
| FLASH
POINT |
154
C |
| STABILITY |
Stable
under ordinary conditions |
|
GENERAL
DESCRIPTION & APPLICATIONS
|
| Formamide, HCONH2 is an amide derived from formic acid, the simplest and lowest
mole weight carboxylic acid in which a single hydrogen atom is attached to the
carboxyl group (HCOOH). Amide is a group of organic chemicals with the general
formula RCONH2 in which a carbon atom is attached to oxygen in solid bond and
also attached to an hydroxyl group, where R is hydrogen in formamide. Formamide
is an transparent hygroscopic oily liquid with an ammonia odor. It is soluble in
water and alcohol; melting point 2.5 C, boiling 210 C. Formamide is obtained by heating ethyl
formate with ammonia or by heating ammonium formate with urea. Formamide is used
in the synthesis of medicine, pesticide, animal glue and other organic compound. It is used
as a solvent which ionizes water-insoluble compounds. It is used as a softening
agent for paper and fiber. It is widely used in the fields of the
spice, dye, plastic as well as textile industries. |
| SALES
SPECIFICATION |
|
APPEARANCE
|
Clear liquid |
|
PURITY
|
99.5%
min
|
|
COLOR,
APHA |
10
max
|
| METHYL
FORMATE |
0.1% max |
| FREE
ACID |
0.01% max |
|
TRANSPORTATION
|
| PACKING |
|
| HAZARD
CLASS |
|
| UN
NO. |
|
| OTHER
INFORMATION |
| Hazard Symbols: T, Risk Phrases: 61, Safety
Phrases: 45-53 |
