ISOBUTANOL
PRODUCT IDENTIFICATION
78-83-1
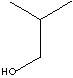
74.12
H.S. CODE
CLASSIFICATION
GENERAL DESCRIPTION
Isobutanol (isobutyl alcohol) is useful in organic synthesis, as a chemical intermediate and as a solvent in coating applications. Isobutanol is similar in property to n-butyl alcohol and may be used as a supplement or replacement for n-butyl alcohol in many applications. As a relatively slow evaporating latent solvent in lacquers and ambient-cured enamels, isobutanol is effective in reducing the viscosities of many formulations while simultaneously promoting flow and retarding blush.
PHYSICAL AND CHEMICAL PROPERTIES
clear liquid
MELTING POINT
-108 C
AUTOIGNITION
413 C
NFPA RATINGS
REFRACTIVE INDEX
APPLICATIONS
Butyl acrylate and methacrylate, glycol ethers, butyl acetate, direct solvent use, plasticizers, amino resins, miscellaneous, including butylamines
APPEARANCE
clear liquid
PURITY
99.5% min
COLOR
10 max, APHA
0.05% max
0.002 max ( KOH mg/kg)
Protect against physical damage. Store in a cool, dry well-ventilated location, away from any area where the fire hazard may be acute. Outside or detached storage is preferred. Separate from incompatibles. Containers should be bonded and grounded for transfers to avoid static sparks. Storage and use areas should be No Smoking areas. Use non-sparking type tools and equipment, including explosion proof ventilation. Containers of this material may be hazardous when empty since they retain product residues (vapors, liquid); observe all warnings and precautions listed for the product.
TRANSPORTATION
1120
GENERAL DESCRIPTION OF ALCOHOL
Alcohols are widely used as solvents, fuels and chemical raw materials. Generally, hydroxyl group compounds are polar, which trends to promote solubility in water. But the carbon chain resist to solubility in water. Short chain alcohols (methanol, ethanol, and propanol) in which the hydroxyl group predominates are miscible in water. Butanol is moderately soluble because of the balance between the two opposing solubility trends. Higher alcohols are practically insoluble in water because of the hydrocarbon chain's trend is stronger. Alcohols are "protic" solvents. Protic refers to a hydrogen atom attached to an electronegative atom, oxygen. Polar protic solvents are compounds that can be represented by the general formula ROH of which water (H2O), methanol (CH3OH) and acetic acid (CH3COOH) are examples. Water-soluble alcohols, low-molecular weight products, are solvents for the manufacture of coatings, dyes and inks, plastics, flavorings, personal-care products, pharmaceuticals, and cleaners. The higher alcohols, slightly soluble in water or insoluble, can provide the proper balance of target properties when solvent-based solvents are formulated for desired viscosity, flowing and leveling, and curing rate and can be used as coupling agents in waterborne coatings.
Alcohols are very weak acids as they lose H+ in the hydroxyl group. Alcohols undergoes dehydration reaction which means the elimination of water molecule replaced by a pi bond between two adjacent carbon atoms to form alkenes under heating in the presence of strong acids like hydrocloric acid or phosphoric acid. Primary and secondary alcohols can be oxidized to aldehydes and ketones respectively. Carboxylic acids are obtained from oxidation of aldehydes. Oxidation in organic chemistry can be considered to be the loss of hydrogen or gain of oxygen and reduction to gain hydrogen or loss of oxygen. Tertiary alcohols do not react to give oxidation products as they have no H attached to the alcohol carbon. Alcohols undergoes important reactions called nucleophilic substitution in which an electron donor replaces a leaving group, generally conjugate bases of strong acids, as a covalent substitute of some atom. One of important reaction of alcohol is condensation. Ethers are formed by the condensation of two alcohols by heating with sulfuric acid; the reaction is one of dehydration. Almost infinite esters are formed through condensation reaction called esterification between carboxylic acid and alcohol, which produces water. Alcohols are important solvents and chemical raw materials. Alcohols are intermediates for the production of target compounds, such as pharmaceuticals, veterinary medicines, plasticizers, surfactants, lubricants, ore floatation agents, pesticides, hydraulic fluids, and detergents.
Carbinol is a primary alcohol with general formula RCH2OH. In carbinol
nomenclature system, the term of carbinol is methanol itself and other groups
are considered to have replaced one of the methanol hydrogen atoms to describe
larger alcohols as derivatives of carbinol. This nomenclature system is
particularly useful when the groups attached to the methanol carbon are large,
aromatic, and cyclo groups. Benzyl alcohol is
called phenylcarbinol or
benzenecarbinol while benzyl carbinol is phenylethyl alcohol.