| CAS
NO. |
67-63-0 |
|
| EINECS
NO. |
200-661-7 |
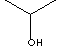
| FORMULA |
(CH3)2CHOH |
| MOL
WT. |
60.09 |
|
H.S.
CODE
|
|
| TOXICITY |
Oral rat LD50:
5045 mg/kg |
| SYNONYMS |
Isopropyl
Alcohol; Dimethylcarbinol;
|
| sec-Propyl alcohol; Rubbing
alcohol; Petrohol; 1-Methylethanol; 1-Methylethyl alcohol;
2-Hydroxypropane; 2-Propyl alcohol; Isopropyl alcohol;
Propan-2-ol; IPA; 2-Propanol; Alcool Isopropilico (Italian);
Alcool Isopropylique
(French); I-Propanol
(German); I-Propylalkohol (German); Iso-Propylalkohol (German); |
| DERIVATION |
|
|
CLASSIFICATION
|
|
|
GENERAL
DESCRIPTION
|
|
Isopropanol
is a clear and flammable liquid at room temperature
with odour resembles that of a
mixture of ethanol and acetone; completely miscible with water, ethanol, acetone,
chloroform, and benzene; melting at -89 C and boiling at 82 C. It undergoes all
chemical reactions typical of secondary alcohols. It reacts
violently with strong oxidizing agents. In a fire, it may
decompose to form toxic gases, such as carbon monoxide.
It is produced from propene by indirect hydration (strong-acid process) or direct catalytic reduction of acetone.
It is a low
cost solvent in many applications as . Isopropanol is similar to ethyl alcohol in solvent properties and evaporation rate. Its high latent solvent
power for cellulose nitrate, cellulose acetate butyrate and cellulose acetate
propionate, along with its moderate evaporation rate and its complete
miscibility with most solvents, make it useful in lacquers, inks and thinners.
Isopropanol is a mature commodity which is projected to grow at a global rate of
1 to 3 percent per year. IPA consumption
for the production of monoisopropylamine for herbicides (primarily glyphosate)
is expected to be the fastest growing segment, while ketone derivatives used as
solvents in coatings and inks will remain flat or increase only slightly.
Government regulations covering volatile organic compounds have been, and
will continue to be, a major consideration in future planning by IPA producers. IPA
is used also in the production of acetone (oxidation of
isopropanol is now the major source of acetone) and its
derivatives and other chemicals (such as
isopropyl acetate, isopropylamine, diisopropyl ether, isopropyl
xanthate, fatty acid esters, herbicidal esters, and
aluminium isopropoxide). Other uses include the application as a coolant in beer manufacture, a
coupling agent, a dehydrating agent, a polymerization modifier in
the production of polyvinyl fluoride, a foam inhibitor, a de-icing
agent, a preservative, a heat-exchange medium, and in windscreen
wiper concentrates. It is also used as a flavouring
agent and in household and personal care products, pharmaceuticals.
|
|
PHYSICAL
AND CHEMICAL PROPERTIES
|
| PHYSICAL
STATE |
clear
liquid, alcohollike
odor |
| MELTING
POINT |
-88
C |
| BOILING
POINT |
108
C |
| SPECIFIC
GRAVITY |
0.786
- 0.788 (20/20 C)
|
| SOLUBILITY
IN WATER |
Miscible |
| pH |
|
| VAPOR
DENSITY |
2.6 |
|
AUTOIGNITION
|
|
|
NFPA
RATINGS
|
Health: 1; Flammability: 3; Reactivity: 0 |
|
REFRACTIVE
INDEX
|
|
| FLASH
POINT |
35
C |
| STABILITY |
Stable
under ordinary conditions |
|
APPLICATIONS
|
|
Solvent , Chemical Derivatives, Acetone, Household And Personal Care Products, Pharmaceuticals.
|
| SALES
SPECIFICATION |
|
APPEARANCE
|
clear
liquid |
|
PURITY
|
99.8%
min
|
|
MOISTURE
|
0.005%
max
|
|
SPECIFIC
GRAVITY
|
0.786
- 0.788 (20/20 C)
|
|
SOLUBILITY
IN WATER |
Pass
test
|
|
COLOR ,
APHA
|
5
max
|
|
ACIDITY
|
10ppm
max (as CH3COOH)
|
|
PRECAUTION IN HANDLING |
|
Protect against physical damage. Store in a cool, dry
well-ventilated location, away from any area where the fire hazard may be acute.
Outside or detached storage is preferred. Separate from incompatibles.
Containers should be bonded and grounded for transfers to avoid static sparks.
Storage and use areas should be No Smoking areas. Use non-sparking type tools
and equipment, including explosion proof ventilation. Containers of this
material may be hazardous when empty since they retain product residues (vapors,
liquid); observe all warnings and precautions listed for the product. |
|
TRANSPORTATION
|
| PACKING |
|
| HAZARD
CLASS |
3
(Packing Group: II) |
| UN
NO. |
1219 |
| OTHER
INFORMATION |
|
Hazard
Symbols: XI F, Risk Phrases: 11-36-67, Safety Phrases: 7-16-24/25-26 |
|
SOLVENT
FUNCTION OF IPA |
|
FUNCTION |
APPLICATION |
| Process solvent |
-Extraction and purification of natural products, such as vegetable
and animal oils and fats, gums resins, waxes, colours, flavourings,
alkaloids, vitamins, kelp and alginates
-Carrier in the manufacture of food products
-Purification, crystallization and precipitation of organic
chemicals
|
| Coating and dye
solvent |
-Synthetic polymers, such as phenolic varnishes and
nitrocellulose lacquers
-Cements, primers, paints, and inks
|
| Cleaning and drying agent |
-Manufacture of electronic parts, for metals and photographic
films and papers, in glass cleaners, liquid soaps and detergents,
and in aerosols |
| Solvent in
topically
applied preparations |
-Pharmaceutical products: embrocations, massage solutions, such
topically as rubbing alcohol (70% 2-propanol aerosols
-Cosmetics: hair tonics, perfumes, skin lotions, hair dye rinses
and permanent wave lotions, skin cleaners and deodorants, nail
polishes, shampoos
|
| Aerosol solvent |
-Cleaners, waxes, polishes, paints, de-icers, shoe and sock sprays,
insect repellants, hair sprays, deodorants, air-fresheners
-Medical and veterinary products: antiseptics, foot fungicides,
first aid and medical vapour sprays, skin soothers, veterinary pink
eye, wound, and dehorning sprays, house and garden type
insecticides
|
|
|
GENERAL DESCRIPTION OF SOLVENT |
Solvent is a
substance, usually a liquid, that acts as a dissolving agent or that is capable
of dissolving another substance. In solutions of solids or gases in a liquid,
the liquid is the solvent. In all other homogeneous mixtures (i.e., liquids,
solids, or gases dissolved in liquids; solids in solids; and gases in gases.),
solvent is the component of the greatest amount. The minor proportion substances
are called solutes. The solvent offers several functions during a chemical
reaction. It solves the substance that reacts with another one to produce a new
set of substances (reactant) and the compound that supplies the molecule, ion,
or free radical which is considered as the attacking species in a chemical
reaction (reagent). The solvent is conductive to collisions between the
reactants and reagents to transform the reactants to new products. The solvent
also takes roll of temperature control, either to provide the energy of the
colliding particles for speedy reaction and to absorb heat in exothermic
reaction. The appropriate solvent should be selected based on the inactivity in
the reaction conditions, dissolving the reagents as well as reactants,
appropriate boiling point and easy removal at the end of the reaction. he most
common solvent is water. Other common solvents which dissolve substances that
are insoluble (or nearly insoluble) in water are acetone, alcohol, formic acid,
acetic acid, formamide. BTX, carbon disulfide, diemthyl sulfoxide, carbon
tetrachloride, chloroform, ether, tetrahydrofuran, furfural, hexane and
turpentine. They may be classified as polar and nonpolar types. They may be
classified as polar and nonpolar types. Polar solvents, like water, have
molecules whose electric charges are unequally distributed, leaving one end of
each molecule more positive than the other. Usually polar solvent has O-H bond
of which water (HOH), methanol (CH3OH) and acetic acid
(CH3COOH) are examples.
Propanol, butanol, formic acid, formamide are polar solvents. Dipolar solvents
which contain a C-O solid bond without O-H bond are acetone
[(CH3)2C=O],
ethyl acetate (CH3COOCH2CH3), methyl ethyl
ketone, acetonitrile, N,N-dimethylformamide and diemthyl sulfoxide. Nonpolar
solvents, like carbon tetrachloride (CCl4), benzene
(C6H6), and diethyl ether (
CH3CH2OCH2CH3), have molecules
whose electric charges are equally distributed and are not miscible with water.
Hexane, tetrahydrofuran and methylene chloride are nonpolar
solvents.
|
![]()