MALEIC ANHYDRIDE
PRODUCT IDENTIFICATION
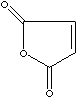
C4H2O3
H.S. CODE
C1(OC(=O)C=C1)=O
CLASSIFICATION
Carboxylic acid anhydride
EXTRA NOTES
UN2215 [Corrosive]
PHYSICAL AND CHEMICAL PROPERTIES
White crystals
1.48
AUTOIGNITION
NFPA RATINGS
REFRACTIVE INDEX
EXTERNAL LINKS & GENERAL DESCRIPTION
Drug Information Portal (U.S. National Library of Medicine) - Maleic anhydride
PubChem Compound Summary - Maleic anhydride
IPCS INCHEM - Cyclic acid anhydrides
Drug Bank - Maleic anhydride
KEGG (Kyoto Encyclopedia of Genes and Genomes) - Maleic anhydride
http://www.ebi.ac.uk/ - Maleic anhydride
http://www.ncbi.nlm.nih.gov/ - Maleic anhydride
Local:
Maleic
Anhydride is the anhydride of cis-butenedioic acid (maleic
acid) which carboxylic acid groups are next to each
other in the cis form. Maleic Anhydride has a cyclic
structure with a ring containing four carbon atoms and
one oxygen atom. It is soluble in acetone, hydrolyzing
in water. It is prepared in commerce by the oxidation of benzene
with catalyst at high temperatures or by the
reaction of C4 (butane) with oxygen in the presence of vanadium catalyst. It is used in 1,4-cyclo
polyaddition and polycondensation as a dienophile. Maleic
Anhydride's biggest single use is in the manufacture
of unsaturated polyester resins for use in fibre-reinforced
plastics in the automotive, construction, marine, consumer
goods and agricultural industries. Producers are working
at capacity, but maleic supplies are barely adequate
for market requirements due to planned and unplanned
downtime in recent days and continued strong demand.
Maleic Anhydride has attractive molecule structure in chemistry. Its reactivity of the two carbonyl groups and the solid bond in conjugation with the two carbonyl oxygens provide broad applications in commerce. Examples of reactions which maleic anhydride are :
- Acylation
- Alkylation
- Amidation
- Cycloaddition
- Decomposition and Decarboxylation
- Diels-Alder reaction
- Electrophilic Addition and Nucleophilic Addition
- Ene Reaction
- Esterification
- Formation of Acid Chloride
- Grignard Reactions
- Halogenation
- Heterogeneous catalytic reduction
- Hydration and Dehydration
- Hydroformylation
- Isomerization
- Ligation
- Michael Addition
- Ozonolysis and Oxidaton
- Polymerization
APPLICATIONS: Unsaturated polyester resins, lubricating oil additives, copolymers, fumaric acid, agricultural chemicals, malic acid, sulfosuccinic acid esters, alkenyl succinic anhydrides and alkyd resins.
APPEARANCE
White crystals
PURITY
99.5% min
SOLIDFICATION POINT
52.40 C min
MOLTEN COLOR
25 max APHA
TRANSPORTATION
2215
HAZARD OVERVIEW
Moisture sensitive. Harmful if swallowed. Causes eye and skin burns. Causes digestive and respiratory tract burns. May cause allergic respiratory and skin reaction. Corrosive to metal in aqueous solution. Sublimes (goes directly from solid to vapor form) readily at room temperature. Target Organs: Lungs, respiratory system, eyes, skin.
GHS
Danger
PICTOGRAMS



HAZARD STATEMENTS
H302-H314-H317-H334
P STATEMENTS
P261-P280-P305 + P351 + P338-P310
![]()
RISK PHRASES
22-34-42/43
SAFETY PHRASES
22-26-36/37/39-45