PRODUCT IDENTIFICATION
H.S. CODE
TOXICITY
CLASSIFICATION
EXTRA NOTES
PHYSICAL AND CHEMICAL PROPERTIES
yellowish crystals
MELTING POINT
SOLUBILITY IN WATER
REFRACTIVE INDEX
EXTERNAL LINKS & GENERAL DESCRIPTION
PubChem Compound Summary - 2,4,6-Trihydroxyacetophenone
KEGG (Kyoto Encyclopedia of Genes and Genomes) - 2,4,6-Trihydroxyacetophenone
http://www.ebi.ac.uk/ - 2,4,6-Trihydroxyacetophenone
http://www.ncbi.nlm.nih.gov/ - 2,4,6-Trihydroxyacetophenone
Local:
Acetophenone, the simplest aromatic ketone, is a clear liquid or crystals;
melting point 19 - 20 C; boiling point 202 C; very slightly soluble in water.
Commercial acetophenone can be obtained from benzene with
acetic anhydride or acetyl chloride by Friedel-Crafts process.
It can be also obtained by air oxidation
of
ethylbenzene, as a by-product of cumene
or from acrylonitrile. It
is used as a polymerization catalyst for the manufacture of olefins. It is used
as an intermediate for pharmaceuticals, agrochemicals and other organic
compounds. It also has been used as a drug to induce sleep. Its is used in tear
gas (especially as the form of chloro acetophenone) and warfare. It is used as
an solvent for plastics, resins, cellulose ethers, and esters. The dimer
(dipnone) is used as a plasticizer. Actophenone and its derivatives, having
additionally substituted saturated alkyls, oxygenated alkyl groups, thio groups,
additional aromatic groups, unsaturated aliphatic side chains, and other
functional groups, are ingredients of flavor & fragrance for in soaps,
detergents, cosmetics, and perfumes as well as in foods, beverages, and tobacco.
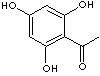
Trihydroxyacetophenone is used as building block for the synthesis of target compounds.
APPEARANCE
ASSAY
99.0% min
MELTING POINT
219 - 221 C
HAZARD OVERVIEW
GHS
PICTOGRAMS

HAZARD STATEMENTS
H315-H319-H335
P STATEMENTS
P261-P305 + P351 + P338
![]()
RISK PHRASES
36/37/38
SAFETY PHRASES
24/25