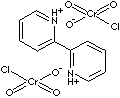
2,2'-BIPYRIDINIUM CHLOROCHROMATE
PRODUCT IDENTIFICATION
CLASSIFICATION
PHYSICAL AND CHEMICAL PROPERTIES
AUTOIGNITION
NFPA RATINGS
REFRACTIVE INDEX
APPLICATIONS
APPEARANCE
97.0% min
OTHER INFORMATION
|
|
|
|
2,2'-BIPYRIDINIUM CHLOROCHROMATE |
||
|
PRODUCT IDENTIFICATION |
||
| CAS NO. | 76899-34-8 |
|
| EINECS NO. | 278-569-1 | |
| FORMULA | C10H10Cl2Cr2N2O6 | |
| MOL WT. | 429.10 | |
| H.S. CODE | ||
| TOXICITY |
|
|
| SYNONYMS | Clorotrioxocromato de hidrógeno, composto con 2,2'-bipiridina; | |
| Hydrogen chlorotrioxochromate(1-) , compound with 2,2'-bipyridine; Chlorotrioxochromate d'hydrogène, composé avec 2,2'-bipyridine; | ||
| RAW MATERIALS |
|
|
|
CLASSIFICATION |
|
|
|
PHYSICAL AND CHEMICAL PROPERTIES |
||
| PHYSICAL STATE | orange to brown powder | |
| MELTING POINT | ||
| BOILING POINT | ||
| SPECIFIC GRAVITY | >1.0 | |
| SOLUBILITY IN WATER | ||
| pH | ||
| VAPOR DENSITY | ||
|
AUTOIGNITION |
|
|
|
NFPA RATINGS |
Health: 2; Flammability: 0; Instability: 0; Special Hazard: OX | |
|
REFRACTIVE INDEX |
||
| FLASH POINT | ||
| STABILITY | Stable under ordinary conditions. Hygroscopic. | |
|
APPLICATIONS |
||
| Chromium does not occur by itself in nature but always in compounds. The stable oxidation states of chromium are +6, +3 (most stable), and +2. In industry, chromic acid refers to chromium(Ⅵ) oxide or chromium trioxide (CrO3). This substance decomposes above 250 C to chromic oxide and oxygen. Chromium in the +6 (or Ⅵ) oxidation state (hexavalent chromium) is a strong oxidant and reacts violently with combustible and reducing materials. The solution in water is a strong acid, it reacts violently with bases and is corrosive. In organic synthesis, chromic acid is used to oxidize primary or secondary alcohols to aldehydes (or ketones) by oxidation state change from +6 to +3. It is difficult to stop at the aldehyde stage during the oxidation reaction, which usually proceeds to the carboxylic acid. Chromate oxidizers such as pyridinium chlorochromate are used to stop at the aldehyde group. The stable oxidation states +3 is the most stable. Saturated primary alcohols are oxidized to carboxylic acids in dimethylformamide solution. Jones reagent (sodium dichromate in aqueous sulfuric acid) continues the oxidation to the carboxylic acid product. Chlorochromates are efficient oxidizing agent for alcohols, allylic and benzylic methylene groups, and for oxidative cleavage of aryl substituted olefins. Hexavalent chromium solutions will not oxidize a tertiary alcohol. Chromium Ⅵ compounds have a dark orange to red color while chromium Ⅲcompounds are normally green. In medicinal field, they are used as anti-infective agents | ||
| SALES SPECIFICATION | ||
|
APPEARANCE |
orange to brown powder | |
| ASSAY |
97.0% min |
|
| TRANSPORTATION | ||
| PACKING | ||
| HAZARD CLASS | 5.1 (Packing Group: II) | |
| UN NO. | 1479 | |
|
OTHER INFORMATION |
||
| Hazard Symbols: O T N, Risk Phrases: 43-49-50/53-9, Safety Phrases: 53-17-26-27-36/37/39-45 | ||
|
|
|