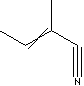
PRODUCT IDENTIFICATION
20068-02-4 (cis)
30574-97-1 (trans)
H.S. CODE
TOXICITY
CLASSIFICATION
PHYSICAL AND CHEMICAL PROPERTIES
MELTING POINT
- 12 C
122 C
SOLUBILITY IN WATER
AUTOIGNITION
NFPA RATINGS
REFRACTIVE INDEX
FLASH POINT
GENERAL DESCRIPTION & APPLICATIONS
Although nitriles don't have a carbonyl group, they are often considered as derivatives of carboxylic acids. Nitrile undergoes acid hydrolysis to form a carboxylic acid. Nitrile is reduced to form amine in the presence of nickel catalyst. Grignard reagents add to nitriles, forming a relatively stable imino derivative which can be hydrolyzed to a ketone. Metaloimine is hydrolyzed to give beta-ketoester. Nitrile undergoes a sequence of nucleophilic additions with an alcohol under acid catalysis, called nitrile alcoholysis. Nitriles are hydrolyzed to carboxylic acids, alcoholyzed to esters, reduction to amines, cyclizized to pyridine derivatives.
2,3-Dimethylacrylonitrile finds applications in dye and flavour & fragrance manufacturing, rubber chemicals, and lubricant as well as a monomer.APPEARANCE
95.0% min (2-Methyl-2-butenenitrile + 2-Methyl-3-butenenitrile)
5.0% max
HCN
30ppm max
Hazard Symbols: F XN, Risk Phrases: 11-20/22, Safety Phrases: 16-23