PRODUCT IDENTIFICATION
868272-85-9 (Tetrahydrochloride hydrate)
H.S. CODE
TOXICITY
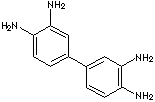
c1(c2cc(c(N)cc2)N)cc(c(N)cc1)N
CLASSIFICATION
Stain, Dye, Aniline, Biphenyl
EXTRA NOTES
A precursor to polybenzimidazole fiber.
PHYSICAL AND CHEMICAL PROPERTIES
MELTING POINT
SOLUBILITY IN WATER
REFRACTIVE INDEX
> 200 C
EXTERNAL LINKS & GENERAL DESCRIPTION
USA.gov - 3,3'-Diaminobenzidine
Wikipedia Linking - 3,3'-Diaminobenzidine
Google Scholar Search - 3,3'-Diaminobenzidine
U.S. National Library of Medicine - 3,3'-Diaminobenzidine
PubChem Compound Summary - 3,3'-Diaminobenzidine
KEGG (Kyoto Encyclopedia of Genes and Genomes) - 3,3'-Diaminobenzidine
ChEBI (http://www.ebi.ac.uk/chebi/) - 3,3'-Diaminobenzidine
NCBI (http://www.ncbi.nlm.nih.gov/) - 3,3'-Diaminobenzidine
Material Safety Data Sheet - 3,3'-Diaminobenzidine
EPA - Substance Registry Services - 3,3'-Diaminobenzidine
Local:
Benzidine is the trival name for 4,4'-diaminobiphenyl which two hydrogen atoms on biphenyl molecules are replaced by amines at para position each. It is a white to slightly reddish crystalline powder turns dark on exposure to air and light; melting point of 128 C. This compound decomposes on heating and reacts violently with strong oxidants, particularly nitric acid. It is produced by the action of acids on diphenyldrazine. Benzidine can be classified as arylamine which one or more of the hydrogen atoms are replaced by aromatic groups such as homologues of aniline, p-aminobiphenyl, benzidine, and naphthylamine. Some arylamines are carcinogenic and the uses are restricted. Benzidne forms a blue precipitate with hemoglobin. Hemoglobin catalyzes the oxidation of benzidine to a polymer by hydrogen peroxide, which gives the blue color. This was the common screening test for occult blood. But the use is restricted due to cancer suspect. Benzidine is the starting material in the dye manufacturing. Numerous substituted group on the benzidine ring system as well as amine groups offer important roles to each characteristic colours directly and in preparing next target colorants. It is important in azo dyes production due to strong fix to cotton. It and its derivatives have active applications in the synthesis of dyes, pigments, paints, rubber compounding agents and wide range of organic chemicals. Biphenyl structure of benzidine also provide the application in luminescence chemistry, spectrophotometric analysis, molecular chemistry, and as a stating material for organometallic-complexes. Benzidine molecules are used in dye production industry and certain other industrial fields. Benzidine molecules include:
APPEARANCE
CONTENT
97.0% min
MELTING POINT
173 ~ 178 C
HAZARD OVERVIEW
GHS (Globally Harmonised System) Classification: Germ cell mutagenicity. Carcinogenicity. Hazard statements: Obtain special instructions before use. Use personal protective equipment as required. IF exposed or concerned: Get medical advice/ attention. Suspected of causing genetic defects. May cause cancer.
GHS
PICTOGRAMS

HAZARD STATEMENTS
H315-H319-H335-H341-H350
P STATEMENTS
P201-P281-P308 + P313
![]()
RISK PHRASES
45-68
SAFETY PHRASES
53-45