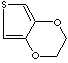
3,4-ETHYLENEDIOXYTHIOPHENE
PRODUCT IDENTIFICATION
126213-50-1
Oral rat LD50: 615 mg/kg
CLASSIFICATION
PHYSICAL AND CHEMICAL PROPERTIES
yellowish liquid
1.334
2.1 (g/l at 20 C)
360 C
104 C
APPLICATIONS
Thiophene, also known as thiofuran, is a cyclic compound containing four carbon atoms and one sulphur atom in a ring. It is a toxic, flammable, highly reactive, colorless liquid insoluble in water (soluble in alcohol and ether) and melts at 38 C, boils at 84 C. It is used as a solvent and chemical intermediate. Its derivatives are widely used in manufacturing dyes, aroma compounds and pharmaceuticals. They are used as monomers to make condensation copolymers.
Due to extended pi-electron cloud overlaps, organometallic molecules or aromatic oligometers such as anthracene exhibit semiconductor properties. Conductive polymers have extended delocalized bonds that creates electrical conductivity when charge carriers generated make positive charges (holes) and negative charges (electrons) move to opposite electrodes. Doping is the intentional impurities in a pure semiconductor to generate charge carriers. The transportation of charges is responsible for fluorescence and electrical energy. These can form well-ordered thin crystalline films. Organic semiconductors have some merits of self radiation, flexibility, light weight, easy fabrication, and low cost. Organic electroluminescence materials have lead to the rapid development of photovoltaic and display devices such as organic solar cells, biosensitizers, OLED(Organic Light Emiting Diode), OTFT(Organic Thin Film Transistor), Wearable Display, and e-Paper. Some examples of organic electroluminescence materials are:- Oligomer Electro Luminescence Materials
- 8-hydroxyquinoline aluminum
- Anthracene
- Pentacene
- Penyl substituent cyclopentadiene derivatives
- Phthaloperinone derivatives
- Perylene derivatives
- Rubrene
- Polymer Electro Luminescence Materials
- Polyanilines
- Poly(p-phenylenevinylene)s
- Poly(thiophene)s
- Poly(alkylfluorene)s
- Poly(acetylene)s
3,4-Ethylenedioxythiophene is a monomer for the manufacture of conjugated polymer [ poly(3,4-ethylenedioxythiophene)] for the applications of redox activity, electroactivity, and conductivity. Other thiophene monomers or oligomers include;
APPEARANCE
yellowish liquid