| Sulfonic acid is
a compound with general formula RSO2OH, where R is an
aliphatic or aromatic hydrocarbon. It is a derivative of sulfuric acid
(HOSO2OH) where an OH has
been replaced by a carbon group or a compound where a hydrogen atom has been
replaced by treatment with sulfuric acid; for example, benzene is converted to
benzenesulfonic acid (water-soluble). Sulfonic acid has a sulfur atom bonded to
a carbon atom of a hydrocarbon and bonded also to three oxygen atoms, one of
which has been attached to a hydrogen atom. Sulfonic acid is acidic due to the
hydrogen atom, stronger than a carboxylic acid. Sulfonic acid is one of the most
important organo sulfur compounds in organic synthesis. Sulfonic acids are used
as catalysts in esterification, alkylation and condensation reactions.
Sulfonates are salts or esters of sulfonic acid. Sulfonic salts are soluble in
water. Sulfonic acid and its salts present in organic dyes provide useful
function of water solubility and or improve the washfastness of dyes due to
their capabiltity of binding more tightly to the fabric. They are widely used in
the detergent industry. Alkylbenzene sulfonic acid is the largest-volume
synthetic surfactant because of its relatively low cost, good performance, the
fact that it can be dried to a stable powder and the biodegradable environmental
friendliness. Sulfonate cleaners do not form an insoluble precipitates in hard
water. Sulfonic acid salts and esters are intermediates widely used in organic
synthesis and particularly phenolic compounds and cation exchange resins. They
are synthetic intermediates for a number of biologically active compounds and
pharmaceutical candidates such as sulfa drugs. Benzenesulfonic acid consumption is linked mostly to phenol and
resorcinol production with
sodium hydroxide. It is used as a catalyst for dehydration and used in
solidifying resins. It is a base material for electroplating solutions.
Benzenesulfonic acid, or a derivative thereof, is used as a synthetic
intermediate for a number of chemical families of pharmaceuticals, pesticides,
dyes, pigments, fluorescent brighteners, and other organic compounds. Commercially, benzenesulfonic acid sodium salt is more common due to high
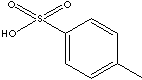
deliquescence of the base material. Toluene sulfonic acids are used in the production of toluenesulfonamide which
has been used as the parent material for the production of saccharin. Large
amount of toluenesulfonamides (mixture of o/p-isomers) is used as a raw material
of flow-promoting agents for paints, hot-melt adhesives, nitrocellulose, coating
materials, thermosetting resins and phenolic resins ans as a basic material of
electroplating solutions. Toluene sulfonic acids are used in preparing hydrazine
based blowing agents such as p-Toluenesulfonylhydrazide, p,p'-Oxybis(benzenesulfonylhydrazide), p-Toluenesulfonyl acetone hydrazone. Toluene
sulfonic acids and their derivatives are used as intermediates for the synthesis of isocyanate
compounds used as water scavengers and catalysts for the production of
thermosetting resins. They are synthetic intermediates for a number of
biologically active compounds, pharmaceuticals, herbicides, dyes and pigments candidates.
Toluenesulfonate esters are useful for the application as alkylating agents in
organic synthesis. p-Toluenesulfonic acid is used as a non-oxidizing catalyst
in the manufacture of plasticizers It is used as a curing agent for
epoxy-phenolic resins. Toluene sulfonic acids are used in preparing hydrazine
based blowing agents such as p-Toluenesulfonylhydrazide, p,p'-Oxybis(benzenesulfonylhydrazide), p-Toluenesulfonyl acetone hydrazone.
p-Toluene
sulfonic acid is often used as a catalyst in the formation of acetal which water must be removed from the reaction mixture to
escape reversible reaction. Water is removed azeotropically
by distilation. (Toluene is the solvent). |